The complexities of the human placenta
DOI: 10.1063/PT.3.5216
Before birth, you were nourished by a unique and extraordinary organ: the placenta. That fetal organ formed an interface between the nutrient-rich blood in your mother’s body and your own, without the two blood supplies ever mixing. The interface supported your growth in the womb, where it effectively served as your lungs, gut, kidney, and liver.

A newborn with umbilical cord and placenta. (From N. Hoboken, Anatomia Secundinæ Humanæ Repetita, Aucta, Roborata, …. [The anatomy of the second human repeated, enlarged, strengthened], Johannem Ribbium, 1675; courtesy of the Thomas Fisher Rare Book Library, University of Toronto.)
The placenta is unique in being a short-lived but versatile organ. Modern imaging techniques reveal its complex microstructure, which is intimately connected to its primary role in exchanging solutes between mother and fetus. Computational image-based modeling helps scientists understand how the intricate spatial organization of maternal and fetal blood vessels influences that exchange and how disease can disrupt it. This article provides a tour of the organ and of physics-based approaches to understanding its life-sustaining role.
Anatomy
Many anatomists throughout history, including Leonardo da Vinci, William Harvey, and Nicolaas Hoboken (discussed in box
The anatomists’ perspective
Leonardo da Vinci is renowned for his detailed anatomical drawings. In his depictions of the placenta, he described the interface between maternal and fetal blood as interwoven hands, and correctly suggested that the two circulations were separate. The prevailing view of early philosophers and many anatomists—from ancient Greek texts through 18th-century scientific literature—was, however, that the maternal arteries of the uterus and fetus enjoyed a direct connection at the placenta.

Seventeenth-century anatomists, such as William Harvey and Nicolaas Hoboken, questioned that view, just as da Vinci had, but they did not have the tools to confirm the functional separation of the two circulations. William Hunter is credited with that achievement. He described how wax injected into the uterine circulation did not appear in the fetal circulation; likewise, wax that he injected into the umbilical vessels did not enter the uterus. He presented his findings in his detailed anatomical atlas Anatomia Uteri Humani Gravidi Tabulis Illustrata; The Anatomy of the Human Gravid Uterus Exhibited in Figures, published in 1774. For a comprehensive history of scientists’ understanding of the placenta, see ref. . (Drawing by Leonardo da Vinci of a fetus in the womb, ca 1510–13, Royal Library, Windsor Castle/public domain.)
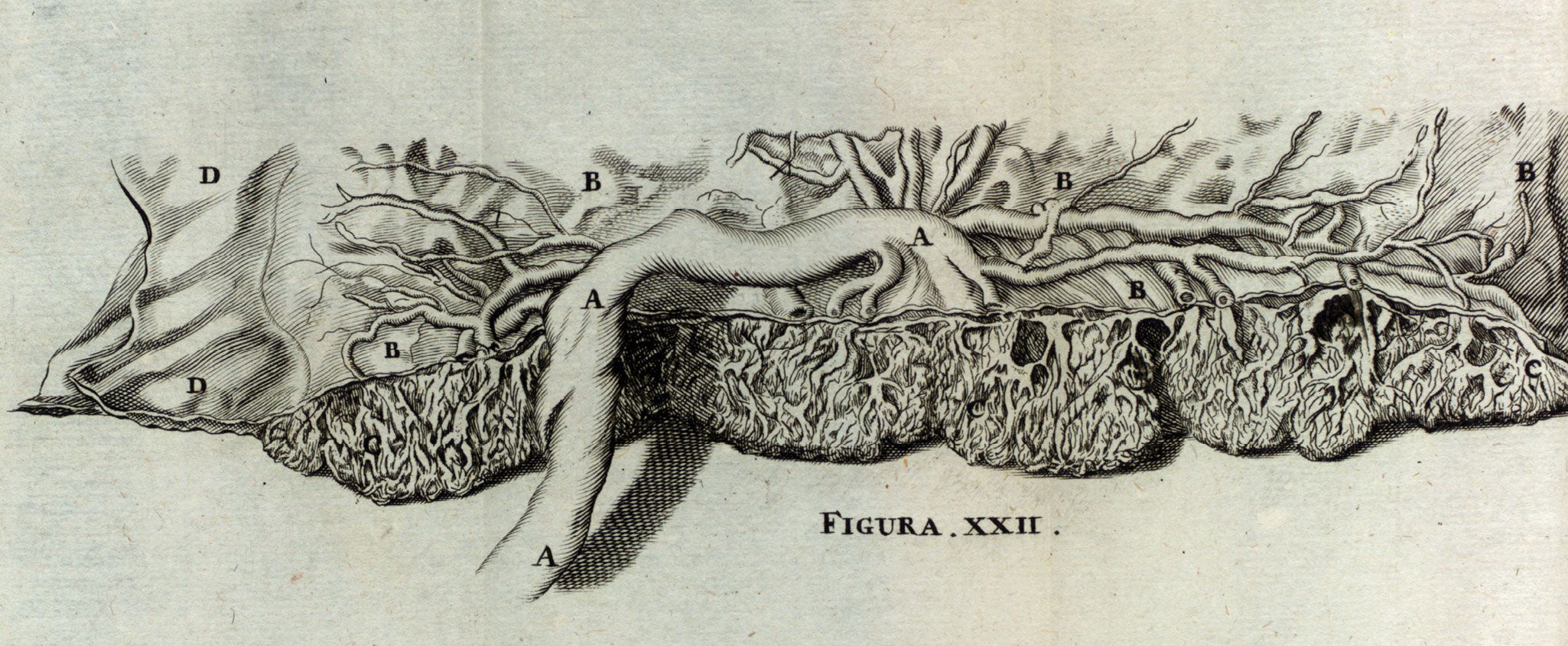
Fetal blood vessels in the placenta occupy complex structures, called villous trees, shown in figure
Figure 1.

The placenta, illustrated. Fetal blood vessels radiate from the umbilical cord (A) over the upper surface of the placenta (the chorionic plate, B) and enter villous trees (C) beneath it. The fetal side of the placenta is protected by an amniotic membrane (D). (From N. Hoboken, Anatomia Secundinæ Humanæ Repetita, Aucta, Roborata, …. [The anatomy of the second human repeated, enlarged, strengthened], Johannem Ribbium, 1675; courtesy of the Thomas Fisher Rare Book Library, University of Toronto.)
A unique, multinucleated cell known as the syncytiotrophoblast covers the entire surface of the placenta, providing a barrier between maternal and fetal blood. The fetal blood vessels themselves branch out from the umbilical cord, over the fetal-facing surface of the placenta, and into the placental tissue in the villous trees. Millions of capillaries sit close to the surface of the placenta, where nutrients and waste are exchanged with maternal blood, as shown in figure
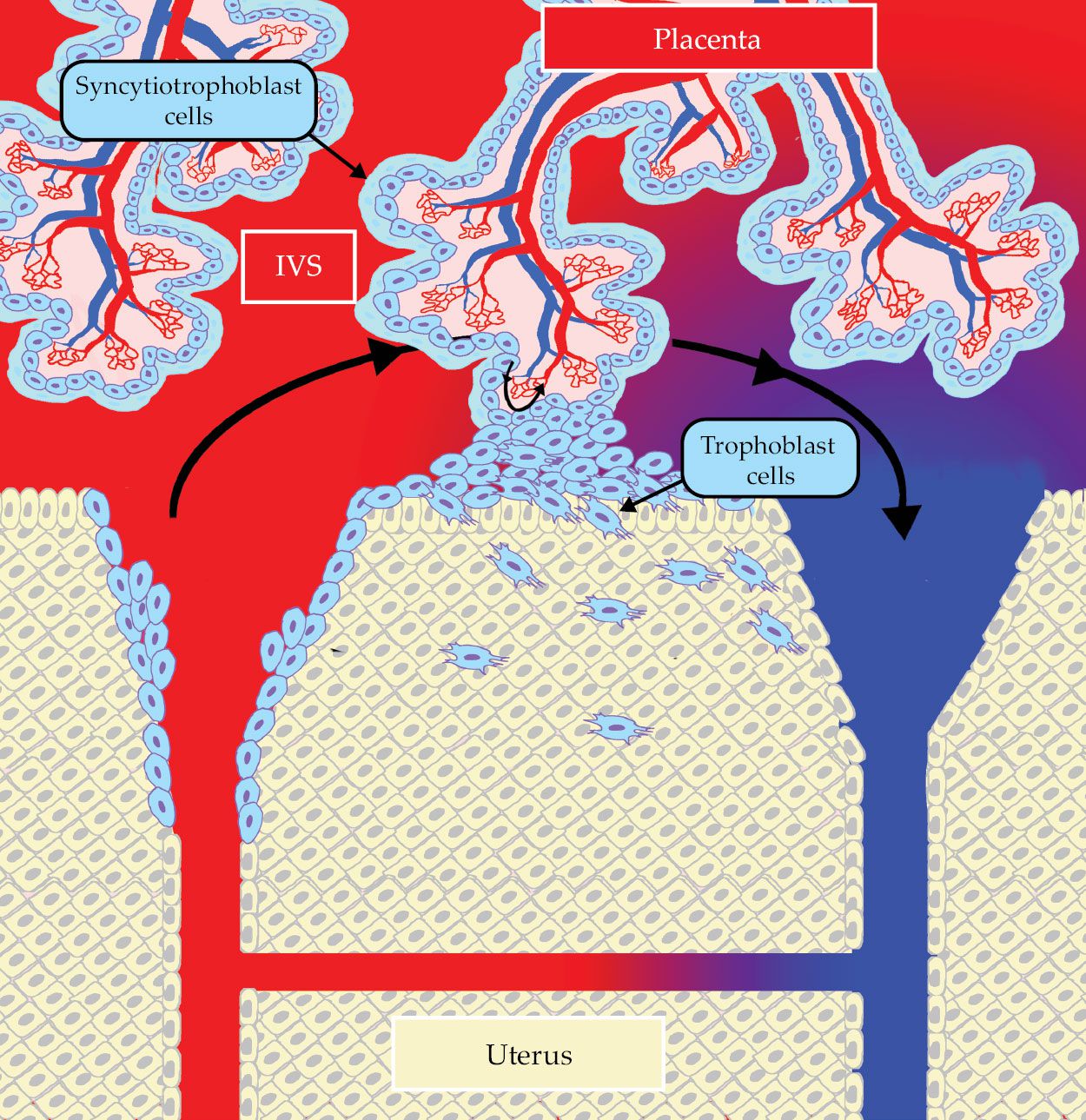
Figure 2.

Oxygenated, maternal blood (red) flows from a spiral artery (left) in the wall of the uterus into the intervillous space (IVS). There, it flows past villous trees in the placenta before being collected in a nearby decidual vein (blue), having lost its oxygen to fetal blood flowing in capillary networks in the villous trees. The maternal and fetal circulations are separated by the syncytiotrophoblast (light blue) and other placental tissues, including trophoblast cells (blue ovals) that invade and remodel the wall of the uterus. Arrows show the flow directions of maternal and fetal blood. A shunt vessel, illustrated in the lower part of the image, connects the uterine artery and vein.
To establish a successful pregnancy, not only must the villous trees develop effectively, but the placenta must also ensure a good blood supply to its surface from the uterus. That process is extraordinary: Placental cells invade the uterine wall and transform the smallest blood vessels into wide nonmuscular channels that allow a significantly increased supply of blood to flow in late pregnancy. (Physicians estimate that the flow increases 15-fold compared with the amount of blood supplied to the nonpregnant uterus.) Even blood vessels that are not invaded by placental cells increase in size—up to twofold—and their behavior is modulated by pregnancy hormones.
The success of a pregnancy therefore relies on the establishment of two blood-supply systems—one fetal (the placenta) and one maternal (the uterus)—in addition to the development of the fetus itself. The interface between the two circulations is of critical importance. They need to be in close contact for effective exchange of solute molecules, but it is dangerous if the blood supplies mix or potentially harmful compounds are transferred to the fetus. Disruptions to blood flow in either circulation can affect the structure of the placenta and the exchange barrier between maternal and fetal blood.
For scientists to understand pathologies of the placenta, they need to have a genuine comprehension of what is normal. That varies widely between human pregnancies and even more so between species. Some pathologies are unique to humans, in part because placentation has evolved to be remarkably different between placental mammals. Accessing the placenta in vivo is challenging because physicians cannot look inside a pregnant mother daily, nor can they use techniques that expose the uterus to ionizing radiation. The placenta is delivered at the end of pregnancy, however, which allows for studies of its structure and function outside the body.
Because the placenta is inaccessible during an ongoing pregnancy, it’s important for scientists to determine what keeps it healthy. We can readily observe snapshots of anatomical structure in delivered placentas, but we need tools to link those snapshots to the drivers of function and dysfunction in the nine months before delivery. Physics-based models help link structure to function and therefore help physicians understand what to look out for in clinical practice, where routine ultrasound scans provide low-resolution insight into placental function during pregnancy.
Pathologies
Several pathologies that relate to the placenta significantly affect the success of a pregnancy and have potentially lifelong effects on the baby. 1 The biggest risk for a pregnancy is stillbirth, which has devastating consequences for the families involved. And the greatest contributor to that risk is a condition known as fetal growth restriction (FGR), whereby a fetus does not grow as well as it should. The pregnancy complication known as preeclampsia is often associated with elevated maternal blood pressure and a poorly adapted circulation in the uterus, and it often accompanies FGR. If untreated, preeclampsia can lead to other complications, including premature delivery and eclampsia—the development of seizures during pregnancy—which is dangerous to both mother and fetus.
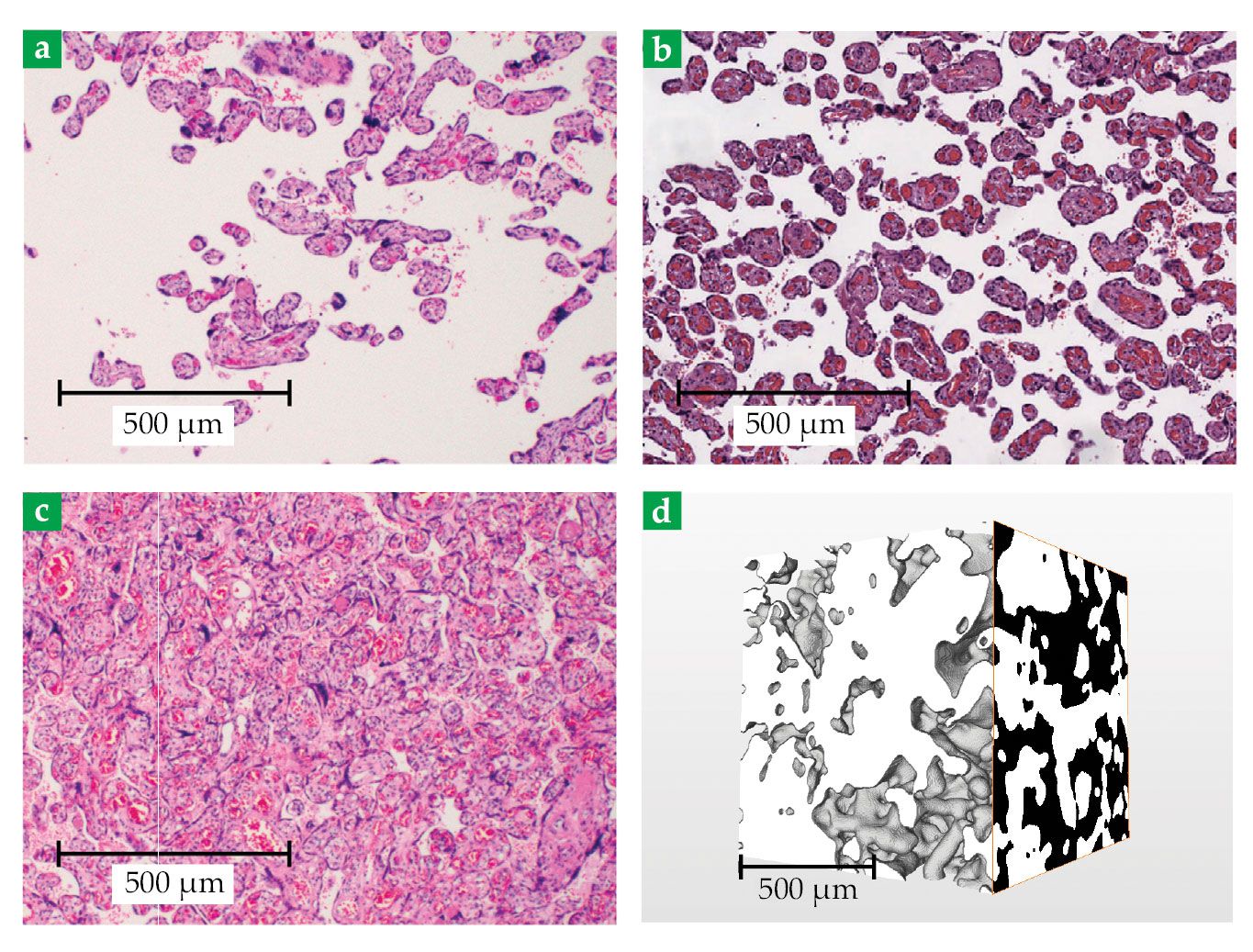
Both FGR and preeclampsia are associated with a reduced density of villous structures in the placenta, as compared with the density of those in a normal placenta (see figures
Figure 3.

Placental tissue. Three cross-sectional images show the intervillous space (IVS; beige) and villous trees (dark spots). Those structures illustrate the typical features of (a) preeclamptic, (b) normal, and (c) diabetic placentas. (Adapted from ref.
Pregnancy complications are notoriously difficult to predict. Problems in low-risk pregnancies need to be picked up early to ensure that any concerns are monitored effectively. Such monitoring reduces the risk of stillbirth in many cases because it allows decisions, such as when to deliver, to be made at the appropriate time. To detect problems early, physicians need a solid understanding of what processes can produce a pathological pregnancy and a way to spot them with the clinical tools available. Despite significant progress, replicating the many roles of the placenta remains a significant technological challenge (see box
A premature lamb grown in a bag
The mystery of childbirth has always fascinated humans. Still, from the time of Leonardo da Vinci to the present, our management of pregnancy and its complications has not changed dramatically. The exceptions involve assisted reproductive technologies, such as in vitro fertilization.
Recently, however, reproductive bioengineering has moved even further afield and begun supporting pregnancies with premature delivery. In 2017, for example, Emily Partridge, Marcus Davey (both at the Children’s Hospital of Philadelphia Research Institute), and their colleagues developed an artificial, fluid-filled womb, or incubator, from which they delivered a healthy lamb. Starting as an extremely premature fetus, the lamb was successfully grown outside the maternal body for nearly a month in the incubator, shown here. 17
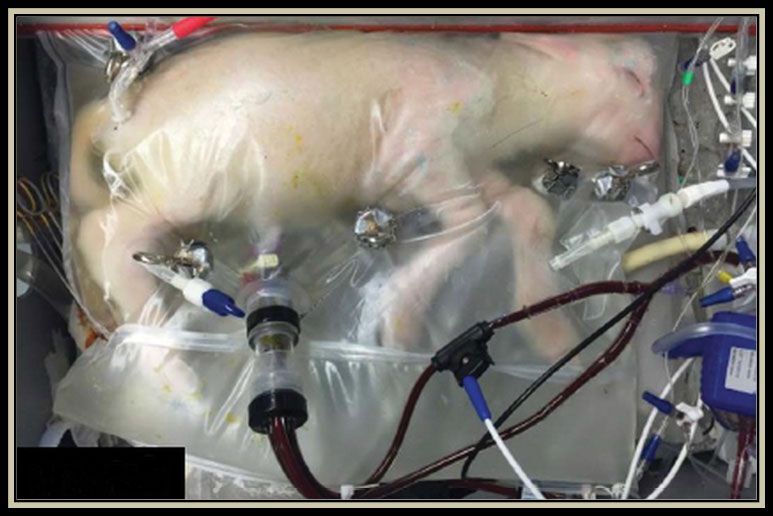
Although those nascent reproductive technologies do not resemble their depiction in Aldous Huxley’s Brave New World (1932), proof-of-concept artificial wombs suggest that the poor outcomes of premature birth could be mitigated. Many practical challenges remain, however, including the design of efficient and robust solute-exchange systems that do not overburden the delicate fetal or neonatal heart. 6 With a deeper understanding of the unique aspects of the human placenta, researchers may be able to develop artificial-womb and placenta technologies to help rescue premature babies and ensure healthy outcomes.
Imaging
Examining an evolving human placenta requires various complementary imaging techniques. 2 , 3 Traditional light and electron microscopy and ultrasound imaging have recently been supplemented by magnetic resonance imaging and micro-computed tomography. 4–6 Synchrotron x-ray tomography of soft biological tissues resolves structures across four orders of magnitude—from microns to centimeters. Applying synchrotron x-ray tomography to the intricate multiscale architecture of the human placenta is opening new windows into placental pathology and providing new opportunities for image-based modeling. Recent advances in x-ray tomography have been enabled not only by robust sample preparation and improved scanning techniques but also by efficient semiautomated segmentation based on machine-learning algorithms.
Even with access to detailed placental microstructure, however, a gap remains in our understanding of how the physics of flow and transport at the organ scale emerges from the dominant processes at the finest anatomical scales. Furthermore, clinical diagnostic tools, such as MRI and Doppler ultrasound, bring an additional layer of complexity because the interpretation of images relies on the physics of imaging technologies and multiple assumptions about the geometry and physical properties of the probed tissue. Future advances in clinical imaging depend on a new generation of physics-based models that can assimilate data from diverse sources across multiple length scales.
Probing the heterogenous multiscale structure of the human placenta also poses significant challenges for quantifying the uncertainties. Many statistical estimates of geometric and material properties of complex soft tissues are intrinsically scale dependent. 6 Care is needed to identify a representative region of interest and to characterize associated fluctuations in key quantities—for example, specific volumetric and surface-area densities or structural-correlation length scales.
Exchange physics
Small molecules, such as oxygen and carbon dioxide, readily diffuse across the syncytiotrophoblast (see figure
Suppose that it takes a typical time TS for a particular solute to cross the syncytiotrophoblast, either by diffusion or active transport. Fetal blood delivered to a terminal villus will spend a typical time TV, say, passing through its capillary network before being returned to the larger conducting vessels of the fetoplacental circulation.
If TV is sufficiently small compared with TS, then diffusive or active transport across the syncytiotrophoblast regulates exchange; in that case, exchange is often called “diffusion limited.” If TS is much smaller than TV, then the rate at which solute is delivered to the fetus is regulated by how fast the blood flowing in the capillaries can carry it through the villus. In that case, exchange is called “flow limited.” 8
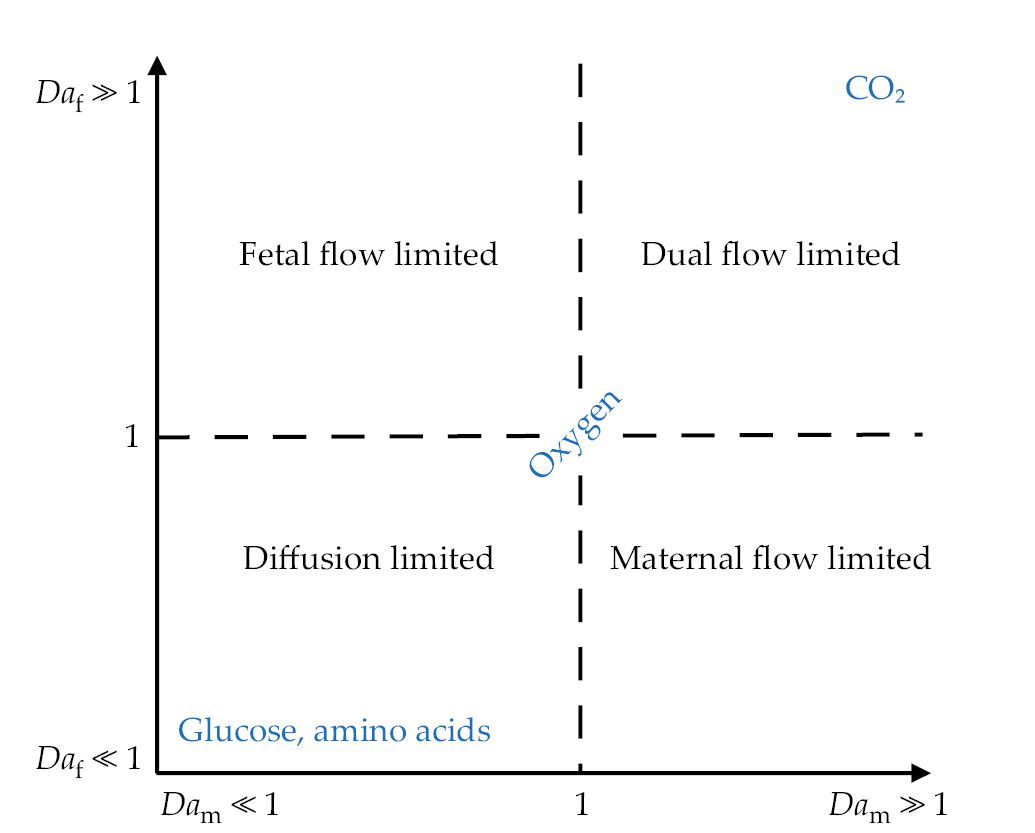
One can characterize the way in which a given solute moves across the walls of a terminal villus by using a dimensionless number, the fetal Damköhler number Daf, defined as the ratio of advective to diffusive fluxes in a typical villus (see figure
Figure 4.

The placenta transports multiple solutes simultaneously, with different physical processes regulating the rate at which they pass between mother and fetus. Small molecules, such as carbon dioxide, diffuse quickly across the syncytiotrophoblast, so that exchange rates are regulated by flow conditions in both the maternal and fetal placental circulations—so-called dual flow-limited transport. The transport of large molecules, such as amino acids, is regulated by the speed at which they cross the syncytiotrophoblast—so-called diffusion-limited transport. The regimes are mapped out here using two dimensionless numbers: the maternal and fetal Damköhler numbers Dam and Daf, respectively. The strength of transport by maternal and fetal blood flow is proportional to 1/Dam and 1/Daf, respectively. Typically, oxygen exchange is regulated by both flow and diffusion. (Adapted from ref.
The maximum solute flux that a villus can absorb in the diffusion-limited state can be expressed as LvDt ΔC, where Lv is a length scale that captures the complex geometric structure of the villus, Dt is a diffusion coefficient in fetal tissue, and ΔC is the difference between fetal and maternal concentrations. The length scale Lv can be interpreted, crudely, as the typical surface area for exchange divided by the typical distance over which solutes must diffuse through villous tissue. It is defined by the shape of the villus and the arrangement of capillaries in it. 9
Suppose that it takes a typical time Tm for a packet of maternal blood to pass through the IVS—from spiral artery to decidual vein. If each villus exchanges solutes with a volume Vm of the IVS, then a characteristic rate at which solute can be exchanged is LvDt/Vm, which assumes diffusion-limited transport in the villi.
Comparing that exchange rate with the transit rate 1/Tm defines a dimensionless maternal Damköhler number Dam, which is proportional to TmLvDt/Vm. Once again, diffusion-limited and flow-limited states are defined by the size of Dam: Very small Dam corresponds to fast flow and relatively slow solute exchange, regulated by the exchange capacity of the villi, with maternal blood leaving the IVS before villi have had time to exchange all the solute. Large Dam, by contrast, describes the case when maternal blood flows slowly relative to the exchange rate. That means that solute can be extracted from maternal blood long before it has left the IVS, but with the net flux passing between mother and fetus falling short of the full exchange capacity of the villi.
Solute exchange is therefore regulated by the strength of both maternal and fetal circulations, as mapped in figure
One might imagine the existence of a sweet spot, with both Daf and Dam of order unity, that allows effective transport without excessively rapid flows. A striking feature of placental transport, however, is the variability in Damköhler numbers for different solutes. Smaller molecules, such as CO2, are more likely to be flow limited, whereas larger molecules, such as glucose, are likely to be diffusion limited, with both being transported simultaneously. 9 (That simple picture becomes richer when one accounts for additional biochemical processes, 8 , 10 such as the binding of oxygen to hemoglobin, and when one accounts for the metabolism of solutes by placental tissue itself.)
The complex architecture of the placenta must accommodate the transport of a broad range of solutes rather than be optimized for a single purpose. An important question to ask then is how the transport of all those solutes may be compromised during disease, when both flow and solute exchange are directly influenced by structural factors that affect both the diffusive exchange capacity and the resistance to flow. If villi are packed too densely, as they are in diabetes patients, they present a high resistance to flow. If villi are packed too sparsely, as in preeclampsia patients, the resistance to flow is low, but the diffusive exchange capacity of the placenta is significantly reduced.
A multiscale complex system
The placenta’s function is regulated by its morphology across multiple spatial scales. At the cellular scale, the particulate nature of blood influences its flow properties in fetal capillaries and the smallest pores of the IVS,
6
whereas the thickness of the syncytiotrophoblast, shown in figure
Figure 5.

The placental exchange barrier (a) separates maternal blood in the intervillous space (IVS) and fetal blood in a fetal capillary (FC). The barrier tissue consists of the syncytiotrophoblast (S) that faces the IVS and endothelial cells (E) that line the FCs. Fetal red blood cells (central blobs) have a maximum diameter of about 8 µm. The double-headed arrow illustrates the distance that solutes must travel between maternal and fetal blood. Single-headed arrows identify the maternal- and fetal-facing sides of the syncytiotrophoblast. (Adapted from ref.
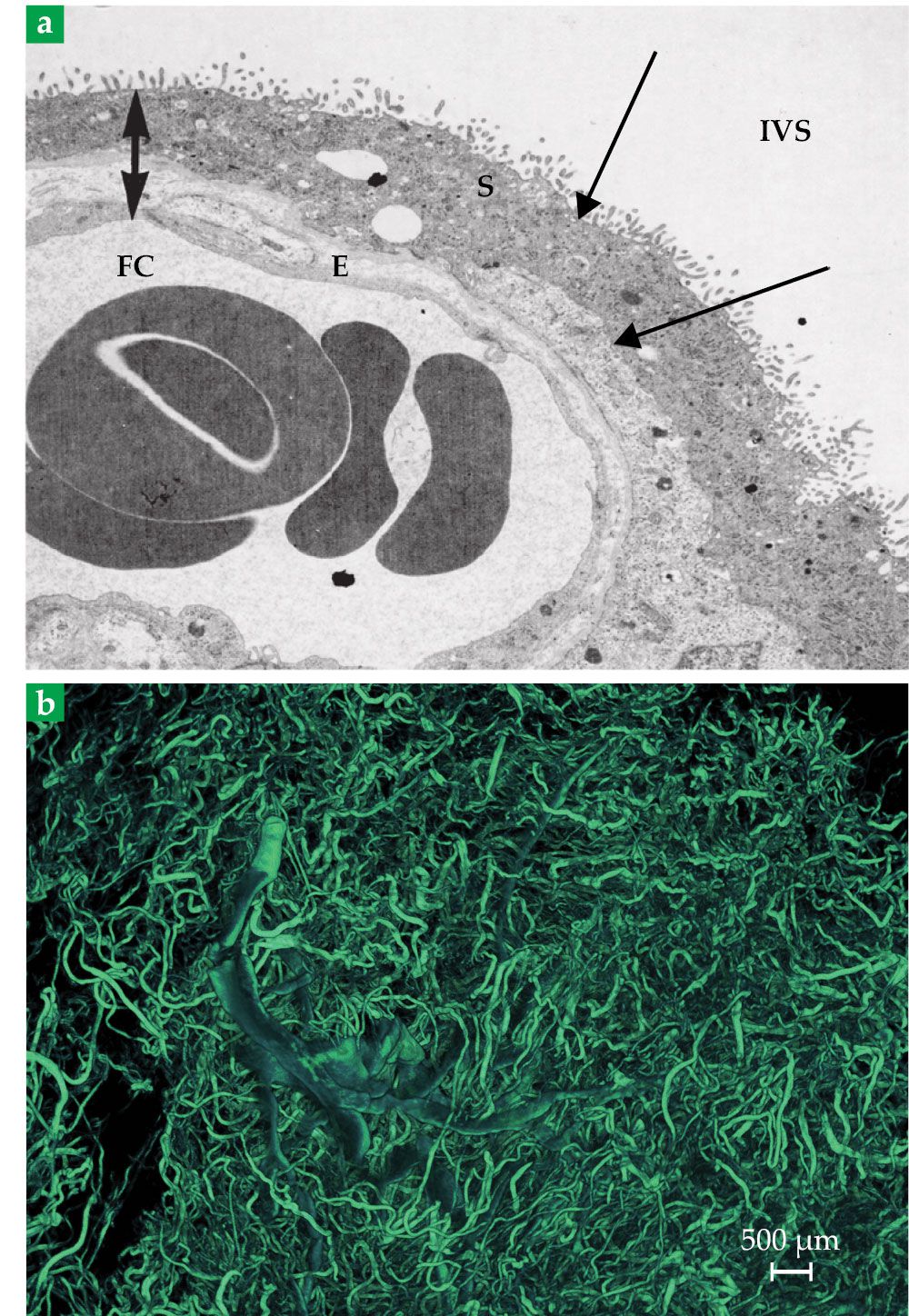
The larger-scale vasculature shown in figure
Homogenization, to date, has mostly been used to simulate blood flow in the IVS, which can be thought of as a disorganized network of pores, through which maternal blood percolates around obstacles—the villous trees. Researchers have widely studied flow through such porous media, particularly in geophysical applications, and have adapted modeling techniques to the placenta. 10
Early studies used two-dimensional cross-sectional images of placental tissue, such as seen in figure
In fetal and uterine circulations, branching blood vessels can be considered mathematically as graphical networks at multiple scales—from disordered capillary networks to large-scale vascular ones. In those models, the flow of blood can be captured using simplified governing equations. The equations do not necessarily incorporate flow disturbances at branching points of the vessels, nor do they explicitly track the movement of oxygen-carrying red blood cells in the vessels. They have, however, provided significant insights into the distribution of blood in the uterus and placenta, and they have been used to identify such features as uterine “shunt pathways,” which are blood vessels that bypass the placenta and directly connect arteries and veins. Network models have highlighted the roles of those pathways in interpreting clinical ultrasound 11 and have paved the way for more in-depth assessment of the uterus to guide future clinical tools.
Because the placenta is structurally so complex, current physics-based assessments make simplifying assumptions to capture its function at different scales of interest. As imaging techniques advance and computational power increases, however, researchers are exploring more complex phenomena in structures representative of the placenta. For example, red blood cells affect both the nature of blood flow in small vessels and the transport biochemistry. (As oxygen carriers, red blood cells must be considered in simulations of exchange capacity.)
In addition, the deformability of the placenta is altered in pathology, but that complexity is rarely considered in simulations of placental function. The compressibility of blood vessels in elastic tissue may have important functional implications when the uterus contracts over the surface of the placenta. 12
The placenta is not the only system determining the health of a pregnancy. Maternal adaptations to pregnancy are important, including the capacity to carry 1 liter per minute of extra blood. The fetus also grows in response to nutrients delivered from the placenta and adapts when it does not get enough.
Scientists are beginning to assess maternal and fetal circulatory health via mathematical models and are developing new tools to noninvasively assess physiology. 12 Maternal and fetal heart rates can be measured at the body surface—with ultrasound or electrodes placed on the stomach—and wearable devices are increasingly being developed that can measure the hearts’ health. The variability in fetal heart rate (and thus cardiac output) can be significant, a factor that affects the amount of blood delivered to the placenta. Understanding the link between the placenta and the fetal circulation that supplies it with nutrients is an ongoing challenge.
What’s ahead?
The mystery of what makes a healthy pregnancy and childbirth is continually being teased out by new technologies. They include well-established medical diagnostic tools, such as ultrasound and MRI, but also machine-learning analyses of images of the placenta and physics-based simulations of how it works. Simulations enable testing of hypotheses and inspire new avenues to investigate.
One of the major challenges in simulating placental function is harnessing the organ’s complex geometry on multiple spatial scales. Researchers need to develop meaningful ways to reduce that complexity and find new strategies for extracting the greatest amount of information from different imaging methods.
Alongside simulation, physics and engineering are providing new tools, such as surface measurements of uterine and fetal electrophysiology, for assessing placental performance and for improving the health of a developing fetus or a newborn via artificial life-support systems. It is likely that the design of those devices and systems could benefit from the results of simulations as well. As scientists from different fields work together, we should see the evolution of new ways to monitor and improve the health of pregnant women and their babies.
Article change history
Updated 13 April 2023: The caption for figure 3 was updated to correctly identify the images of the normal and preeclamptic placentas.
Work on “Multi-modal studies to understand pregnancy and prevent stillbirth” is supported by Wellcome Leap as part of the In Utero Program.
References
1. K. Benirschke, G. J. Burton, R. N. Baergen, Pathology of the Human Placenta, 6th ed., Springer (2012).
2. A. Clark et al., Br. J. Radiol. 95, 20211010 (2022). https://doi.org/10.1259/bjr.20211010
3. R. M. Lewis, J. E. Pearson-Farr, Placenta 102, 55 (2020). https://doi.org/10.1016/j.placenta.2020.01.016
4. M. Byrne et al., J. Theor. Biol. 517, 110630 (2021). https://doi.org/10.1016/j.jtbi.2021.110630
5. J. L. James et al., Placenta 114, 8 (2021). https://doi.org/10.1016/j.placenta.2021.08.049
6. Q. Zhou et al., Curr. Opin. Biomed. Eng. 22, 100387 (2022). https://doi.org/10.1016/j.cobme.2022.100387
7. C. P. Sibley et al., Placenta 64, S9 (2018). https://doi.org/10.1016/j.placenta.2018.01.006
8. J. J. Faber, K. L. Thornburg, Placental Physiology: Structure and Function of Fetomaternal Exchange, Raven Press (1983).
9. A. Erlich et al., Sci. Adv. 5, eaav6326 (2019). https://doi.org/10.1126/sciadv.aav6326
10. O. E. Jensen, I. L. Chernyavsky, Annu. Rev. Fluid Mech. 51, 25 (2019). https://doi.org/10.1146/annurev-fluid-010518-040219
11. A. R. Clark, T. C. Lee, J. L. James, WIREs Mech. Dis. 13, e1502 (2021). https://doi.org/10.1002/wsbm.1502
12. N. S. Dellschaft et al., PLoS Biol. 18, e3000676 (2020). https://doi.org/10.1371/journal.pbio.3000676
13. A. S. Serov et al., J. Theor. Biol. 364, 383 (2015). https://doi.org/10.1016/j.jtbi.2014.09.022
14. W. M. Tun et al., J. R. Soc. Interface 18, 20210140 (2021). https://doi.org/10.1098/rsif.2021.0140
15. M. Desforges, C. P. Sibley, Int. J. Dev. Biol. 54, 377 (2010). https://doi.org/10.1387/ijdb.082765md
16. L. D. Longo, L. P. Reynolds, Wombs with a View: Illustrations of the Gravid Uterus from the Renaissance through the Nineteenth Century, Springer (2016).
17. E. A. Partridge et al., Nat. Commun. 8, 15112 (2017). https://doi.org/10.1038/ncomms15112
More about the authors
Alys Clark is an associate professor of bioengineering at the University of Auckland in New Zealand. Igor Chernyavsky is a Presidential Academic Fellow in applied mathematics and Oliver Jensen is the Sir Horace Lamb Professor in the department of mathematics, both at the University of Manchester in the UK.







