A machine that mechanically interlocks molecules
DOI: 10.1063/pt.ctwr.kguo
Figure 1.

Mechanically interlocked molecules are connected not by chemical bonds but by their shapes, which makes them useful for engineering at the molecular scale. Ring-shaped molecules, represented here by orange and gray circles, can be connected like links in a chain to form what is known as a catenane. Catenanes have been effectively synthesized for decades by using ions, such as copper (green dot), that temporarily hold them in place—a process known as templated synthesis. (Illustration by Freddie Pagani.)

It might be impossible to quantify the number of machines involved in daily life. We use machines to control the climate in our homes, move between places, and heat up water for our morning cup of coffee or tea, among other things. Somewhat less obvious, though, is the fact that our very ability to get up in the morning and make a caffeinated drink relies on a more hidden kind of machinery: molecular machines that convert the energy and carry the signals that power our bodies. Those tiny biological machines serve as inspiration for work that extends human engineering capacity down to the molecular level.
“The molecular scale, obviously, has very different rules than the macroscopic scale. Everything flies around in this Brownian hurricane all the time—everything moves and vibrates and wiggles,” says Michael Kathan of Humboldt University of Berlin. In an environment awash with the noise of thermal motion, directing energy into specific tasks requires different strategies than the ones used in the macroscopic world (see the article by Dean Astumian and Peter Hänggi, Physics Today, November 2002, page 33
An early step was the synthesis of mechanically interlocked molecules. In contrast to covalently bonded atoms, which share valence electrons in a covalent bond, mechanically interlocked molecules are connected by their physical shapes, as shown in figure
Another major advance in the engineering of molecular machines was the formulation of a molecular motor that can spin in one direction. The first molecular motors worked by exploiting shape interactions and energy-absorption differences to drive two sides of a molecule into relative circular motion about the axis of a carbon double bond. For creating the basic components of molecular machines—mechanically interlocked molecules and molecular motors—Jean-Pierre Sauvage, J. Fraser Stoddart, and Bernard Feringa were awarded the 2016 Nobel Prize in Chemistry (see Physics Today, December 2016, page 18
The capabilities of synthetic molecular machines have been demonstrated in various applications: changing the shape of macroscopic materials they are embedded within, moving liquids up a ramp, replicating the movements of more-familiar machines like cranes and cars, and storing data, for example. Now Tommy Wachsmuth and a team of researchers in Kathan’s lab at Humboldt University of Berlin have shown how a molecular motor can be used to build catenanes. 1 It’s a molecular machine that builds the potential components of other molecular machines.
“It’s the first real example of the motion of a molecular machine being connected to a specific bond-forming reaction. Each cycle of operation leads to a different product, not just switching between outcomes,” says Jonathon Beves of the University of New South Wales in Sydney, Australia. The research provides a key demonstration that such machines can be used to do mechanical work at the molecular scale. Molecular motors can twist molecules into thermodynamically disfavored but kinetically stable shapes that could not be made with conventional chemical reactions. The use of molecular machines to create new molecules opens the door to a world of new possibilities in chemical synthesis.
Nanoscale rules
Chemists have been trying to make catenanes since the 1950s and 1960s. Those early efforts produced various approaches that worked, but only with impractically small yields—a few percent at best. In the early 1980s, Sauvage devised a more effective way to build them. He used copper ions as a template to hold ring- and crescent-shaped molecules together. A subsequent chemical reaction would close each crescent to form interlocked loops, like those shown in figure
Figure 2.
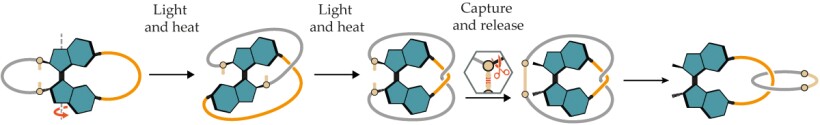
A molecular motor (shown in blue) can be used to wind two hydrocarbon chains (shown in gray and orange) attached to its rotors. After a 360° turn, the chains cross twice. Chemical reactions can then be used to connect one of the chains to itself (tan line), which captures the interwound state, and then sever the chain’s chemical bond to the motor to release it. The result is a mechanically interlocked molecule, a catenane, that could not have been made with conventional chemical reactions alone. (Figure adapted from ref.
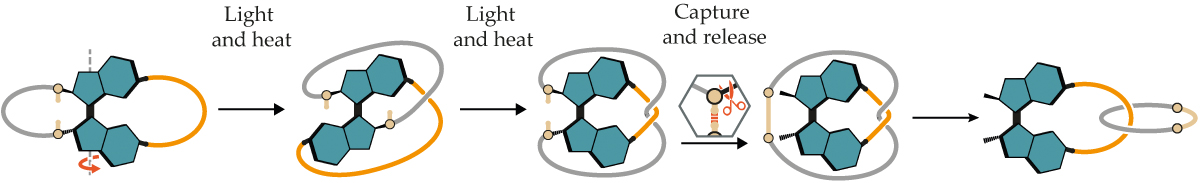
Using a motor to twist molecules into interlocked rings is a totally new approach. The molecular motor used by Kathan’s team is essentially the same as the first ones built by Feringa in 1999. The motor is made of overcrowded alkenes—large molecules with a carbon double bond connecting two sets of branching lobes.
The lobes are large enough that they can’t all sit in a single plane. Shining a specific wavelength of light on the molecule causes the double bond to flip, and the molecule takes on a higher-tension, metastable shape. The addition of heat then provides enough energy for what’s known as thermal helix inversion—the molecule swivels to a stable shape. Those two steps produce a 180° turn, as shown in figure
That design paved the way for researchers to make motors with constant, speedy rotation by tweaking the alkene substituents and applying heat and light together. But for the task of winding molecules together into catenanes, the Kathan lab turned back to the first-generation design, in which high activation barriers provide exquisite control over every half turn of the motor.
The research team connected hydrocarbon chains as tethers to the motor’s rotors, as shown in figure
Though a 360° turn was enough to build a catenane, the tethered motors could be turned as far as 720° to generate two crossings. Attempts to capture that doubly wound state were unsuccessful, though, because the molecule would spontaneously turn back 180°, presumably because of strain in the tethers. Unexpectedly, covalent capture of tethers that had been wound up by 540° produced a higher yield of catenanes than those that had only been turned one full circle: 90% compared with 82%. Both yields, though, were exceptional.
Winding forward
A template-based approach to catenane synthesis doesn’t work for all molecules. One advantage of using a machine to interlock molecules is that it could be used on molecules that don’t have the bonding sites necessary for templated synthesis. To demonstrate that distinct ability, the researchers used the motor to build catenanes out of hydrocarbon strands that, because of their limited number of functional groups, can’t be readily manipulated with the templated method.
One drawback of the technique is that the motor is part of the final product. Unlike highly efficient biological machines, such as ribosomes, which can turn out thousands of proteins, one machine yields only one product, for now. Separating the motor from the second tether isn’t as simple as reproducing the capture and release steps used to sever the first tether. If the researchers had used identical tethers, the symmetry of the molecules would have reduced the selectivity of the process—the asymmetry of the molecular system provides more control over the shape of the final product.
“Recycling is key because the motor is challenging to make,” says Kathan. With the proof of concept in place, the Kathan lab is already looking for ways to separate and reuse the motor while retaining control over the final product.
The exact ways that such molecular motors and catenanes may be put to use, though, remain further off. “On the technological side, we are still far from real-world applications,” says Emanuele Penocchio of Northwestern University in Evanston, Illinois, “but I think the results are promising.”
Regarding the bigger picture of the design and use of new molecular machines, Penocchio says that unlike the development of macroscale technology in the industrial revolution, nanoscale engineering has the advantage of researchers knowing what is possible, because they “have biology that demonstrates it.” (See, for example, the article by Mohammed Kaplan, Physics Today, March 2024, page 28
“Since the synthesis of vitamin B12 by [Robert Burns] Woodward and [Albert] Eschenmoser in the 1970s, we basically know that you can make any organic molecule that you want. But this is by no means true for molecules that have a complex three-dimensional shape or topology,” says Kathan. The high yields and chemical flexibility of the new method are both positive developments for the field. Thermodynamically unfavored molecules can also store energy. But perhaps most notable is the demonstration that molecular motors can be used to direct the synthesis of molecules that otherwise couldn’t be made.
“Where this will lead us is difficult to say,” says Kathan. “But I think biology and also macroscopic machines really set the stage for everything that’s possible.”
This article was originally published online on 19 September 2025.
References
1. T. Wachsmuth et al., Science 389, 526 (2025). https://doi.org/10.1126/science.adx5363
More about the authors
Laura Fattaruso, lfattaruso@aip.org





