Spectroscopy shines a light on an electrode–water interface
Interfacial water—that is, the water that resides in the immediate vicinity of a surface—behaves differently from that of the bulk. Even though it remains liquid, interfacial water near a solid electrode, for example, often forms a layered, ordered structure that shares some properties with ice.
The hydrogen evolution reaction involves two protons and two electrons reacting at a cathode to yield molecular hydrogen. When interfacial water participates in the reaction, positively charged hydrogen atoms dissociate from oxygen ones in an electrolyte solution and react with electrons donated from a nearby electrode to form H2, a potential alternative to fossil fuels.
Now Feng Pan
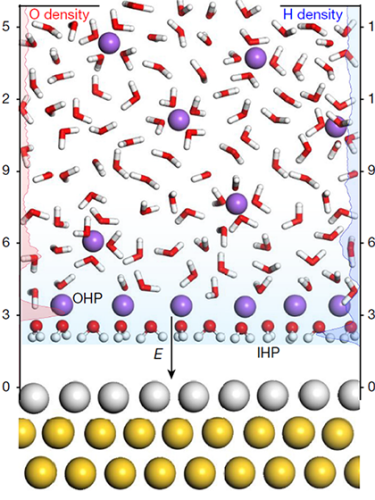
Sodium ions (purple circles) help bring water molecules closer to an electrode surface of palladium (silver circles) layered on gold nanoparticles (yellow circles).
Y.-H. Wang et al., Nature 600, 81 (2021)
To collect their data, the researchers adapted a Raman spectroscopy technique that Li helped design in 2010
The researchers focused on measuring interfacial water by preparing a 50-μm-thick electrolyte solution, which shares little to no behavior with that of bulk water, and trapping it between two electrode surfaces. For their experiment, one of the electrodes consisted of a crystalline surface of palladium sitting atop a layer of gold nanoparticles.
At the water–electrode interface, the researchers applied an electric potential ranging from 0.29 V to −1.11 V. Starting at negative electric potentials, the researchers found that the Raman spectra of the interfacial water includes a broad band of spectral lines centered at 550 cm−1. That band indicates a reciprocating vibrational motion of interfacial water, which previous work found to be related to an ordered water structure. A second band with a Raman shift centered around 3300 cm−1 is indicative of hydrated sodium cations concentrated in the interfacial water region.
Pan and some of his colleagues used density functional theory to learn more about sodium’s contribution to the reaction. Ab initio molecular dynamics simulations found that palladium–hydrogen bonds are shorter in the presence of hydrated sodium. A shorter bond is a stronger one and promotes water dissociation. The simulations show that hydrated sodium may bring water closer to the electrode surface than it would get without the sodium. That proximity, the researchers say, improves the electron transfer efficiency between the electrode and the interfacial water and accelerates the hydrogen evolution reaction. (Y.-H. Wang et al., Nature 600, 81, 2021
More about the authors
Alex Lopatka, alopatka@aip.org




