Soot formation: A new mechanism for an old problem
DOI: 10.1063/PT.3.4061
When they are burned, diesel, kerosene, firewood, and other carbonaceous fuels produce black and brown carbon particles known as soot. Humans have used soot as a pigment since prehistoric times and still use it in tires, inks, and plastics. But soot generally is not humanity’s friend. Unregulated coal-burning led to soot-blackened buildings, like Edinburgh’s St Giles’ Cathedral in figure
Figure 1.

St Giles’ Cathedral in Edinburgh. Unregulated coal burning from the Industrial Revolution up to the 1950s led to many soot-blackened buildings in the UK. (Photograph by Rachelle Burnside/ Shutterstock.com.)

On Earth, no natural processes besides combustion produce soot. In interstellar space, similar high-temperature conditions may produce similar carbonaceous dust (see the article by Alessandra Candian, Junfeng Zhen, and Alexander G. G. M. Tielens on page 38
The reactions that turn gaseous fuel molecules into that layered structure are difficult to measure and extremely rapid. Most measurement techniques alter the conditions under which the reactions proceed, and current models cannot account for the speed at which the reactions occur.
Now Hope Michelsen, Olof Johansson, and colleagues at Sandia National Laboratories, Lawrence Berkeley National Laboratory, and the University of California, Berkeley, have made detailed measurements of molecules in the flames produced by burning different fuels and have identified a chemical pathway of rapid reactions that could explain how soot forms. 1
Possible precursors
The combustion science community has explored two main classes of mechanisms to explain soot inception, the term given to the production of particles from precursor gaseous hydrocarbons. The first comprises physical bonding processes, in which forces such as van der Waals interactions or hydrogen bonding cause planar precursors to aggregate into stacked clusters. Most of the gaseous hydrocarbons typically present, however, have a vapor pressure too high to nucleate by such physical forces at 1400 K, the temperature at which incipient soot particles occur. A long history of research has tried to identify how the molecules could become less volatile. But none of the proposed pathways, such as oxygenation or ionization, could simultaneously bring about nucleation and obey the laws of thermodynamics. 2 Physical bonding alone cannot explain soot inception.
The second class of mechanisms involves macromolecule formation through irreversible chemical reactions. 3 Those processes focus on the growth of stable hydrocarbons consisting of one or more planar ring-shaped structures, from the addition of small species like acetylene, which is a by-product of most common combustion reactions even when it’s not the primary fuel. In the early 1990s, Michael Frenklach and Hai Wang, then at the Pennsylvania State University, proposed that a key reaction involves the removal, or abstraction, of a hydrogen atom from a stable hydrocarbon subunit, followed by an acetylene addition. 4 But the energy required to activate the stable molecule involved in those reactions is very high, so the reactions proceed too slowly to completely explain soot inception.
Experiments have shown the importance of the hydrogen abstraction– acetylene addition reaction in the growth of soot precursors. A 2014 study by Tyler Troy, Musahid Ahmed, and colleagues at Lawrence Berkeley showed that acetylene, when combined in a thermal reactor with a single-ring hydrocarbon, reacted to yield a two-ringed molecule.
5
(See the Quick Study by Troy and Ahmed, Physics Today, March 2015, page 62
Radical reactions
Michelsen and her colleagues now propose a sequence of chemical reactions involving certain molecules that can act as catalysts for growing clusters of hydrocarbons. Using electronic structure calculations, the researchers have proposed that the key to creating soot is a large, self-sustaining pool of a specific kind of radical—a molecule with an unpaired electron. The special radicals gain stability by resonant intramolecular interactions. When one resonance-stabilized radical reacts with another molecule, its free electron becomes paired and energy is released. Then, because of a low energy barrier due to the resonance stabilization, that new molecule loses a hydrogen atom and becomes a resonance-stabilized radical again. The new, bigger radical can bond with another molecule and continue to generate radicals in a chain reaction.
Michelsen and her team looked for evidence of their proposed radicals in different kinds of soot-forming flames, such as the ethylene–oxygen flame shown in figure
Figure 2.

A controlled flame for studying soot. The flame forms at the interface between an upward flowing jet of ethylene fuel and a downward flowing jet of oxygen. The blue color is the result of light emitted by excited small radical species. The yellow is the result of luminescence from hot soot particles. (Image courtesy of Hope Michelsen and Matthew Campbell.)

Because radicals are more reactive than nonradical species of the same mass, they adhered to the incipient soot particles extracted from the flames. Resonance-stabilized radicals are more stable than normal radicals, so they could survive during the sampling and detection process. The technique thus concentrated the resonance-stabilized species at the spectrometer detector. In every case, radicals appeared in the regions of the mass spectrum where no other signals appeared. But for a long time, Michelsen says, “we thought of those radicals as a curiosity. Like almost everyone else, we were focused on finding a mechanism that would make more stable hydrocarbons stick together.”
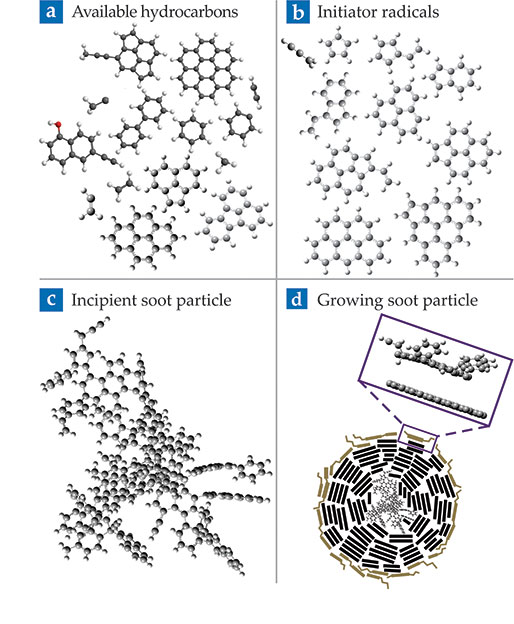
Figure
Figure 3.

Soot formation mechanism. Black circles are carbon atoms, white are hydrogen. (a) Molecular species that are in the flame when soot is formed include small multiple-ring hydrocarbons, small carbon chains, and hydrocarbon radicals. (b) Resonance-stabilized radicals like propargyl in the top left can initiate clustering of other hydrocarbons, such as the multi-ring molecules in panel a. (c) Rapid clustering by the radicals forms incipient soot particles. (d) Reactions between radical sites on the soot particle’s surface (purple inset) and other hydrocarbons lead to rapid particle growth. (Adapted from ref.
Good clean soot
The proposed reactions circumvent the problem of using up radicals that can react quickly because they allow for clustering of a wide range of hydrocarbon sizes and structures. Wang, now at Stanford University, observes that the work “opens an alternative, viable path to a more rational explanation of the hydrocarbon growth phenomenon.” Future research to verify the proposed mechanism will investigate reaction rates and yields of the resonance-stabilized radicals, formation rates and identities of nucleated particles, and mechanisms of growth at the primary particle’s surface.
As the process by which gaseous fuel molecules form soot becomes less mysterious, researchers and engineers expect to look for ways to reduce or control soot emission from engines and industrial processes, all of which are important for both health and climate. Such advances in understanding carbonaceous aerosol formation could have parallel benefits for synthesizing novel carbon materials such as nanotubes and graphene. What’s more, the same formation mechanism could explain how gas in interstellar space produces carbonaceous stardust.
References
1. K. O. Johansson et al., Science 361, 997 (2018). https://doi.org/10.1126/science.aat3417
2. H. Wang, Proc. Combust. Inst. 33, 41 (2011). https://doi.org/10.1016/j.proci.2010.09.009
3. M. Frenklach et al., Symp. (Int.) Combust. 20, 887 (1985). https://doi.org/10.1016/S0082-0784(85)80578-6
4. M. Frenklach, H. Wang, Symp. (Int.) Combust. 23, 1559 (1991). https://doi.org/10.1016/S0082-0784(06)80426-1
5. D. S. N. Parker et al., Angew. Chem. Int. Ed. Engl. 53, 7740 (2014). https://doi.org/10.1002/anie.201404537




