Physical and mathematical approaches yield insights into how cancer develops and spreads
DOI: 10.1063/PT.3.4223
Despite improved prognoses for certain types of cancer, progress in combating the disease has been modest since the declaration in 1971 of the “war on cancer.” Heart disease and cancer are the leading causes of death in the US. And with the nation’s population aging, the number of cancer deaths is expected to continue rising. Aside from cancer’s lethality and enduring mystery, the realization that cells respond to physical and mechanical cues in addition to chemical and genetic ones is drawing physical scientists to study the disease.
Historically, physicists have played a major role in developing diagnostic and treatment tools for cancer—from x rays and MRI to lasers, radiation therapies, and designer drugs. But in the last decade or so, physical scientists and engineers have increasingly delved into understanding the disease, an area that had been almost exclusively the domain of biologists and medical researchers.
By helping to select the most relevant data, bringing more math to analyze them, and creating meaningful theoretical constructs, physical scientists have already contributed significantly to understanding cancer, says Anna Barker, who a decade ago at the National Cancer Institute (NCI) was key in launching the Physical Sciences–Oncology Centers (see Physics Today, November 2014, page 22
Physical scientists study myriad aspects of cancer, including how tissue stiffness correlates with cancer; how cancer cells modify their environment, differ from healthy cells, and migrate, select, and create sites for metastasis; whether proteins act as mechanical–chemical switches; and how to improve prognosis predictions. Researchers use mathematical and computational models, machine learning, engineered systems, tumor cells in two- and three-dimensional cultures, animal subjects, and clinical studies with humans. The wide-ranging studies help develop a fuller picture, says Josef Käs, a biophysicist at the University of Leipzig in Germany. “Cancer is such a complex problem that there is no single $100 million question. But we are understanding more by the day.”

Eliane Blauth, a master’s student in Josef Käs’s lab at the University of Leipzig in Germany, looks at an image of a fluorescently labeled tumor.
HANS KUBITSCHKE

Cancer repurposes healthy cells
One point of entry for physical scientists who want to understand cancer is tumor progression. Kandice Tanner, a physicist at the NCI, uses zebrafish to study organ selectivity in cancer metastasis (see the
Xavier Trepat, a group leader at the Institute for Bioengineering of Catalonia in Barcelona, Spain, focuses on the forces involved in cancer development. In metastasis, for example, cells detach from a primary tumor, move through tissue, travel through the bloodstream or lymphatic system, and eventually form secondary tumors. Each step involves many proteins and genes, says Trepat, but the steps also involve mechanical processes. To enter a blood vessel, a cancer cell has to push other cells out of the way and deform itself. His lab does in vitro experiments to measure the forces in such processes.
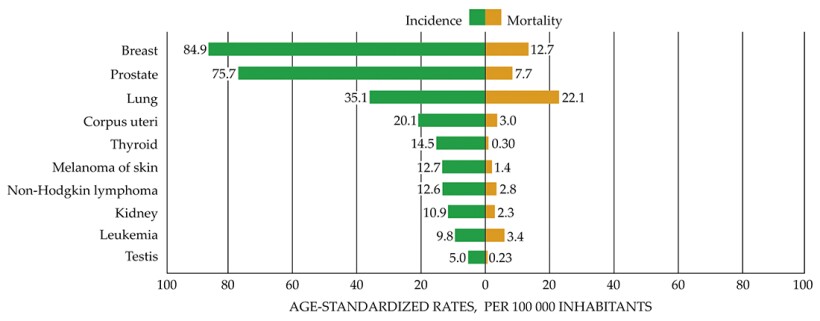
Incidence and mortality rates in the US for different cancer types. (Reproduced with permission from J. Ferlay et al., Global Cancer Observatory: Cancer Today, International Agency for Research on Cancer, 2018. Available from the interactive website https://gco.iarc.fr/today
The 1–100 nN forces exerted by healthy cells enable motion, and they also transmit biological signals—telling a cell to secrete a protein, express a gene, divide, die, or perform another action. Cancer cells commandeer those signals to compel other cells to do what they need.
In his experiments, Trepat mixes tumor and healthy cells, “because any function you look at in a tumor is affected by nontumor cells.” He and his team found that skin-cancer cells are not themselves mobile. Rather, they exploit the mobility of healthy fibroblasts, cells that secrete collagen and other macromolecules to form connective tissue and facilitate wound healing. Cancer cells adhere to fibroblasts and get pulled from the tumor. “These tumor cells hijack the function of fibroblasts to help them migrate,” he says. “This cross talk between healthy and cancerous cells ultimately helps cancer spread.”
Many tumor types are stiffer than normal tissue; cancer is often diagnosed by palpation. Tissue stiffens when the fibers in it become more cross-linked or stretched, or it becomes denser through proliferation of cancer cells. As a tumor grows, it increases the local pressure, which cancer cells may better withstand than normal cells can.
Tissue stiffness can be measured in situ using ultrasound or magnetic resonance elastography. And Viola Vogel and her group at ETH Zürich recently synthesized a peptide that binds only to relaxed tissue fibers. That selectivity allows the researchers to distinguish—for the first time, Vogel says—relaxed from stretched fibers. “Surprisingly, we found that tumor tissues contain a large fraction of relaxed fibers. Since nothing is known about the tension of tissue fibers in healthy and diseased tissues, we need to figure out what that means physiologically.” She adds that the peptide might be used to identify and target diseased fibers remaining after chemotherapy.
Tumors and nearby healthy tissue can be excised and then compared when stretched and strained. Liver-cancer cells, for example, proliferate and lose their liver characteristics more when they are grown on stiff substrates than on softer ones. Dennis Discher, a biophysicist at the University of Pennsylvania who heads one of the NCI’s 12 Physical Sciences–Oncology Centers, says that by the 2000s, researchers understood that a tissue’s softness is important for its health. The risk of liver cancer rises if the organ stiffens, whatever the cause—alcohol, a virus, cirrhosis, fibrosis, or other source.
Stiffness in the extracellular matrix drives cancer progression, says Janine Erler, a cancer biologist at the University of Copenhagen in Denmark. She and physicist Lene Oddershede of the university’s Niels Bohr Institute embed healthy and cancerous cells in collagen matrices and use optical tweezers to measure the cell stiffness as a function of tissue density. The matrix density doesn’t alter healthy cells, says Oddershede, but invasive cancerous cells can become softer or stiffer in response to the stiffness of the matrix. “In a stiffer microenvironment, cells behave more aggressively,” Erler says. What’s not known is whether the matrix stiffness is a driver or a by-product of the aggressiveness.
Only aggressive cancerous cells can squeeze through pores in the surrounding tissue matrix. The pair’s goal is to understand what regulates cell behavior—what cues cells to invade or renders them unresponsive to drugs, says Erler. “If we can understand how a cell senses it’s in a stiff environment, we may be able to change the response. It opens the possibility that we could trick cells into thinking they are in a soft environment.”
Käs and his group in Leipzig observe tumor chunks that have been surgically removed from people with breast and cervical cancers. They have seen areas where more than half the cells are round and don’t move. In between are fluid areas where cells stream to the surface and leave the tumor. It’s like the jamming–unjamming transition in colloids, he says. “Cells can jam and unjam through shape changes. What is becoming clear is that it’s not what the single cell does, it’s collective behavior.” (See, for example, the Physics Today articles by Robert Evans, Daan Frenkel, and Marjolein Dijkstra, February 2019, page 38

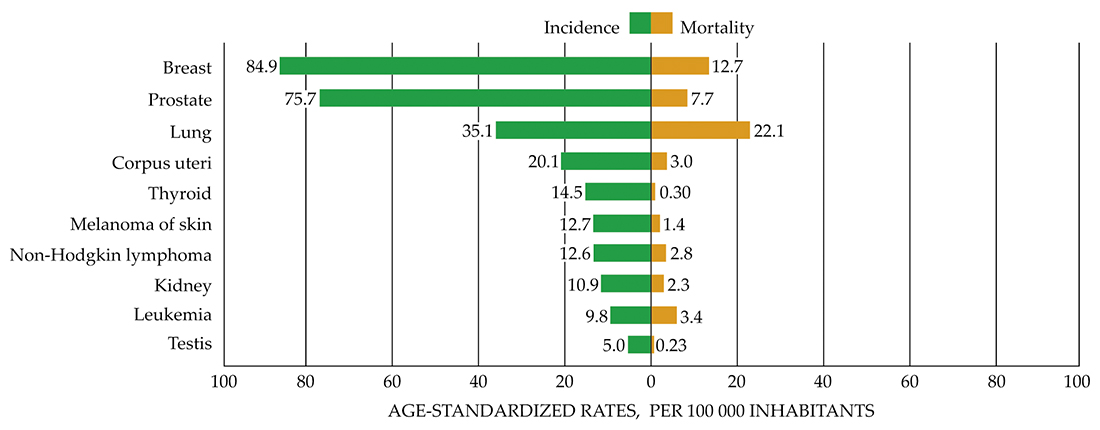
Human breast cancer cells were injected into a zebrafish, where they spread and colonized in the brain (blue).
COLIN PAUL AND KANDICE TANNER
Killer metastasis
Generally, a patient can be cured only when the primary tumor can be fully removed before metastasis occurs. Even then, cancer cells may have spread undetected and the disease may recur. For Robert Austin, a Princeton University physicist, the most pressing question is how cancer metastasizes. “Ninety percent of cancer deaths are due to metastatic cancer,” he says. “That is the linchpin.” (See the article by Chwee Teck Lim and Dave Hoon, Physics Today, February 2014, page 26
Tumors are highly stressed environments: acidic, hypoxic, and low in nutrients. Austin and his group image cell movement on microfabricated silicon substrates while varying conditions such as drug gradients and fluid flow. They mix metastatic cell lines “to get as generic as possible,” he says.
The cells that survive the harshest conditions become polyploid—they engulf other cells and end up with multiple sets of chromosomes from different cell types (see the
Cynthia Reinhart-King of Vanderbilt University’s department of biomedical engineering also uses fabricated systems to study cancer. “We can engineer collagen to look like tumor collagen, and we can adjust the pore size and the fiber size of collagen.” With such systems, she and her group try to find out, for example, how cells move along fibers and why tissue density is a prognostic indicator for breast cancer. “We tailor systems to look at aggressive cells, the role of tissue structure, and what drives cells to move and grow. We study single cells and collective behavior.”
One of the biggest contributions by physicists and engineers, Reinhart-King says, is showing that it’s not just the cells themselves that promote disease. “We have identified that tissue structure, stiffness, porosity, and fluid gradients can all contribute to cancer progression.” In addition, she says, researchers have discovered that conditions in the microenvironment can make normal cells behave like cancer cells and coax cancer cells to behave like healthy ones.
Metastasis involves leader–follower behavior, with some cells showing the way in leaving the primary tumor, says Reinhart-King. She and other researchers have found that energy determines which cells lead, and when those cells run low on energy, others take over. But how individual cells become leaders and followers in the first place remains unclear. And given that a primary tumor can shed thousands of cells a day, why are there relatively few metastatic sites?

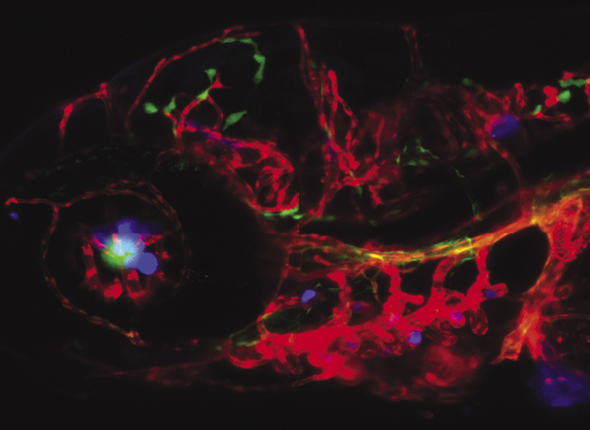
This metastatic prostate cancer cell has more than 30 sets of chromosomes, accumulated from a combination of engulfing foreign nuclei and exposure to a chemotherapy drug. The red dots are fluorescently labeled proteins in the nuclei.
GONZALO TORGA
Predicting prognosis
Herbert Levine, a theorist at Northeastern University, analyzes signaling and decision making in cancer cells. (See the article by Jané Kondev, Physics Today, February 2014, page 31
Theoretical physicist Benjamin Greenbaum, director of the Center for Computational Immunology at Mount Sinai, studies immune-driven tumor evolution and tumor response to therapies that activate or suppress the body’s immune system to fight cancer. Why, for example, does immunotherapy do nothing for one group of patients with metastatic melanoma, but in another it seemingly wipes the disease out? He is also part of an interdisciplinary team, funded by the charity Stand Up To Cancer, that is investigating why a small fraction of patients survive pancreatic cancer for an unusually long time. He and colleagues model the evolutionary dynamics of tumors. They compare a tumor’s molecular alterations, such as arise from mutations, that the immune system can recognize, with the aim of pointing the way to treatments for nonresponders. (Last year’s Nobel Prize in Physiology and Medicine was awarded to James P. Allison and Tasuku Honjo “for their discovery of cancer therapy by inhibition of negative immune regulation.”)
Clare Yu, a theoretical physicist at the University of California, Irvine, uses statistical techniques to analyze the efficacy of immunotherapy for treating triple-negative breast cancer, an aggressive, hormone-independent cancer. From the density and distribution of immune cells in patients, she evaluates the response to immunotherapy and the likelihood of recurrence. “We can predict with about 70% accuracy whether cancer will recur in a patient within five years,” she says.
Yu is also interested in why tumors occur where they do. More than half of breast cancers, for example, are found near the armpit. Twice as many lung cancers start in an upper lobe than in a lower lobe. Colon cancer starts preferentially in the first half of the colon. “It’s not random. It’s a spatial question—a physical property,” says Yu. She likes the question “because it’s not clear you could answer it by signaling pathways. Something other than signals or toxins is implied.”
Princeton’s Austin also applies theoretical approaches to approximate and predict cancer behavior. Coupled nonlinear partial differential equations can describe the interactions between cancer cells and the noncancer cells in connective tissue, he says. “It’s game theory. You can try to get at what the future will be, how the cancer propagates.” Metastasis cannot currently be predicted, he notes. So the value of such calculations would be to estimate outcomes and help prevent overtreatment.
Far more features are visible in cancer tissue than can be identified by eye, says pathologist Roberto Salgado, who works in Belgium and Australia. He integrates machine learning and fractal analysis with spatial genomics, which correlates genetic information in DNA and RNA with location and function of tissue. The shape of cell nuclei, chromatin density, growth patterns of cancer cells, and patterns of blood vessels are some of the variables pathologists use to distinguish between aggressive and more indolent cancers. “Machine learning could complement the work of pathologists,” says Salgado. “I think we need to uncover much more of the architectural complexity of cancer, the hidden secrets of cancer morphology. If we do that and then match the spatial architecture of cancer with the genomics, we can make progress for our patients.”
One key to meaningful progress in cancer research is improved communication among biologists, clinicians, pathologists, and physical scientists. Last year Memorial Sloan Kettering Cancer Center’s Larry Norton and colleagues launched the privately funded Mathematical Oncology Initiative to help build a common language and bring more math to cancer research.
More about the authors
Toni Feder, tfeder@aip.org





