Our bodies store hormone peptides in structures usually thought to be toxic
DOI: 10.1063/1.3226703
Dispersed in the brains of Alzheimer’s patients are disk-shaped lesions about 100 µm across. Whether those lesions, or plaques, are a cause or a consequence of Alzheimer’s disease is controversial, but their composition is clear. The plaques are made from fibrous aggregates—amyloid—of protein or their shorter cousins, peptides.
Amyloid also shows up in the neuropathology of Huntington’s, Parkinson’s, and other pernicious diseases. Once sequestered in amyloid, a protein or a peptide can no longer perform its function. Even if amyloid does not directly cause those diseases, it seems at best a useless, dead-end repository of proteinaceous material.
But as a new paper exemplifies, a less malign view of amyloid is emerging. Roland Riek of ETH Zürich and his collaborators in India, Sweden, and the US have demonstrated that our bodies exploit amyloid as a temporary storage medium for a wide range of hormones, including pain-relieving endorphin and appetite-suppressing bombesin. 1
Riek’s discovery adds to the modest but growing set of examples of so-called functional amyloid that perform useful tasks, among them fixing memory in snails, templating melanin in humans, and transmitting inheritance in yeast. It will also influence a major goal that spans the biological, medical, and physical sciences: determining amyloid’s detailed structure and formation mechanisms (see the article by Clare Grey and Robert Tycko on

Riek began to suspect that peptide hormones were stored as amyloid during an investigation of a hormone called corticotropin-releasing factor, which is secreted by the hypothalamus in response to stress. He observed that CRF aggregates into amyloid fibrils. Moreover, the amino acids that promote aggregation play little role in CRF’s prime function: binding to and activating its receptor. Because aggregation is usually harmful, evolution tends to discard sequences that promote it. Could CRF aggregation be useful, Riek wondered?
Before being released to do their jobs, peptide hormones are stored in small dense capsules known as secretory granules. Electron diffraction and nuclear magnetic resonance had revealed the granules’ contents to have near-crystalline order. Amyloid, while not fully ordered, could qualify as the structure that peptide adopts when packed in granules.
To test the idea, Riek and his team subjected 42 peptide hormones to a battery of biochemical, biophysical, and crystallographic tests. First they determined whether the peptide hormones spontaneously form amyloid under the conditions that prevail in granules: high density and an acidic pH of 5.5. Only 10 did, but a further 21 hormones formed amyloid in the presence of a molecule, heparin, implicated in granule formation.
Storing hormones as amyloid is not much use if the hormones can’t be unpacked when needed. Riek and his team therefore immersed the hormone amyloid in a solution whose pH of 7.4 matches the mildly alkaline conditions hormones typically encounter when they’re released in vivo. The amyloid duly dissolved, breaking apart into its constituent peptides.
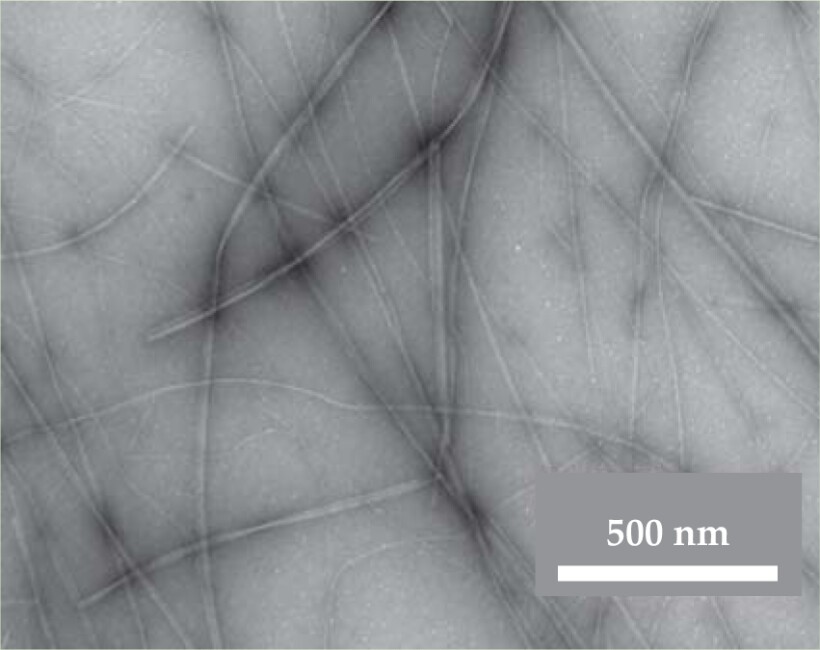
In those tests, Riek’s team detected the presence of amyloid by using Congo red and other dyes that bind to amyloid. More direct evidence comes from looking for amyloid fibrils in electron micrographs. All the peptide hormones formed fibrils, including β-endorphin, shown here in the top figure. X-ray diffraction provides the definitive test. Amyloid fibrils exhibit order on two principal length scales, 4.7 and 9.3 Å. Those scales appeared in the diffraction patterns.
Having shown that hormone peptides form and unform amyloid in vitro, Riek’s team applied a final test: to determine whether hormone peptides are stored as amyloid in vivo. The lower figure shows the result of applying two stains: Thio S, which stains amyloid green, and an antibody that is engineered to stain a particular peptide red—in this case, mouse growth hormone. Features stained by both appear yellow, indicating that growth hormone and the others are indeed stored in mice as amyloid.
How could amyloid have emerged as a storage medium? Many proteins include one or more structural elements called β strands. When the protein first folds up, β strands pleat in a zigzag fashion to form a β sheet like a stored fire hose. In folded proteins, the hydrophobic β sheets are found inside, away from the protein’s water-facing surface. Amyloid fibrils, as first recognized by William Astbury in 1935, are made up of stacked β sheets, as if the aggregated proteins had been opened up, allowing the sheets’ hydrophobic surfaces to avoid contacting water by sticking together. Astbury boldly proposed that most proteins naturally exist as either folded-up singletons or amyloid aggregates, depending on external conditions.
Conditions that promote amyloid formation appear rarely in organisms. It’s possible, therefore, that β strands evolved to support protein folding and that functional amyloid evolved as nature happened on, and then controlled, amyloid-promoting conditions. As for the role of amyloid in disease, evidence is growing that the precursors to amyloid—few-molecule strings of protein—are the toxic agents, not amyloid itself. Those oligomers would indeed be toxic if their normally hidden β sheets are exposed. Amyloid plaques, then, would represent a secured waste dump of toxic material.
References
1. S. K. Maji et al., Science 325, 328 (2009). https://doi.org/10.1126/science.1173155




