Nickel oxide superconductor has cuprate-like electronic properties
At first glance, layered neodymium nickel oxide doesn’t seem like a strong superconductor candidate. Although its structure resembles that of calcium copper oxide, the parent compound of the cuprate superconductors, it does not share fundamental CaCuO2 properties such as antiferromagnetism and insulating behavior. So it came as somewhat of a surprise last year when researchers at SLAC and Stanford University synthesized a strontium-doped NdNiO2 compound that superconducts below about 15 K (see Physics Today, November 2019, page 19
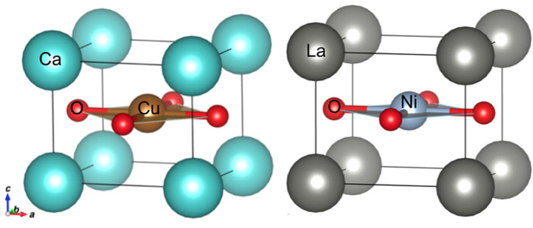
A. S. Botana, M. R. Norman, Phys. Rev. X 10, 011024 (2020)
Via a series of electronic-structure calculations, Botana and Norman compared CaCuO2 and lanthanum nickel oxide (LaNiO2), which has nearly identical properties to the newly discovered nickelate superconductor. The researchers focused on three factors that previous studies have correlated with superconductivity in cuprates. The values of two of those properties—related to d-orbital energy splitting and the distances that electrons can hop within each layer—were found to be nearly identical in both compounds. The results suggest that the two properties are important for cuprate-like superconductivity.
The major distinction between the compounds, the researchers determined, is the energy difference between the p level of the oxygen and the d level of the transition metal. The small value of that charge-transfer energy in cuprates, 2.7 eV in CaCuO2, is thought to promote the Cooper pairing of electrons. At 4.4 eV, the value in LaNiO2 is so high it could suggest that charge-transfer energy is not as crucial a factor for cuprate-like superconductivity. Or perhaps that difference explains the relatively low superconducting transition temperature of NdNiO2 compared with those of the cuprates.
In addition to exploring the factors underlying cuprate-like superconductivity, Botana and Norman hope to predict the superconducting potential of similar nickel compounds. With only the lone nickelate superconductor known so far, the big question is whether NdNiO2 is an anomaly or just one of many in a new family of superconductors. (A. S. Botana, M. R. Norman, Phys. Rev. X 10, 011024, 2020
More about the authors
Andrew Grant, agrant@aip.org




