Mechanical force may determine the final size of tissues
DOI: 10.1063/1.2731960
Even before a fly egg first divides, the structural changes that culminate in an adult fly begin. The body’s two major axes emerge first, followed, in the embryo, by the appearance of compartments that will become the mouth, legs, and other organs of the eventual maggot.
Those structural milestones, and later ones in the life cycles of flies and other organisms, are controlled by signaling molecules called morphogens. Since the 1970s biologists have identified numerous morphogens in their favorite fly, Drosophila melanogaster. The morphogens’ often whimsical names, like wingless and hedgehog, describe deformities that befall the fly when the corresponding genes mutate.
Although their underlying biochemistry is complex, the ability of morphogens to pattern tissue and trigger growth appears to be a straightforward consequence of the response they evoke in cells and of their spatial distribution and transport properties. What’s harder to understand is how—or whether—morphogens determine a growing tissue’s final, correct size.
To grapple with that question, developmental biologists work with fly tissues called imaginal disks. Those simple sheets of epithelial cells form in the heads of maggots. By the time the maggot has become an imago (its first adult stage), the disks resemble the wings and other organs they’ll eventually become. The disks of D. melanogaster are not especially easy to work with. They are small and don’t grow in vitro. But those disadvantages are offset by the preexisting trove of data gathered on the fly’s anatomy, physiology, and genetics.
Viewed under a microscope, the imaginal disk cells appear to divide and grow at a rate that depends only weakly on their location in the tissue. What tells them to stop? The question is all the more puzzling because, as the image on the cover shows, morphogen concentration varies strongly across the disk.
Two years ago Boris Shraiman of the Kavli Institute for Theoretical Physics in Santa Barbara, California, asked himself what could prevent the cell division rate from following the morphogen profile. His answer, in a theoretical paper published two years ago, is mechanical feedback. 1 Shraiman hypothesized that mechanical stress affects cell growth and proliferation through an as-yet unidentified molecular mechanism. In particular, compression acts as a growth inhibitor.
If unchecked, cells under the central peak of a morphogen concentration profile will divide more vigorously than cells under the profile’s wings. To create space to grow, the central cells must push aside the more slowly dividing cells that surround them. That accommodation is easy if cells, which behave mechanically like water-filled balloons, obligingly slide past each other. But if cells resist being parted from their neighbors—that is, if the disk behaves like an elastic solid—pressure builds. Cells in the center will be compressed radially, while cells on the periphery will be stretched laterally.
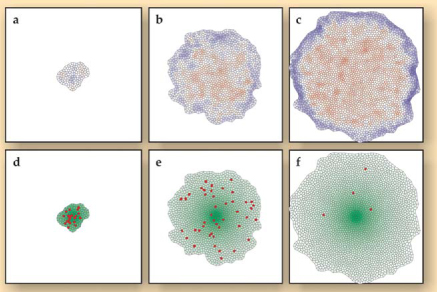
Working with two colleagues, Lars Hufnagel of KITP and Hervé Rouault of école Normale Supérieure in Paris, Shraiman went on to devise a numerical simulation that demonstrated how mechanical feedback could determine disk size. 2 The accompanying figure shows the simulation at the start, middle, and end of the growth cycle.
As the simulated disk grows, mechanical stress builds up in such a way as to compensate for the nonuniform distribution of the growth-promoting morphogens. The result is nearly uniform growth throughout the disk. Growth slows when cells on the periphery get so far from the source of the morphogen that they no longer proliferate. Compression rises in the still-growing center and eventually shuts down growth completely.
When Shraiman proposed mechanical feedback, he didn’t know whether imaginal disks resist being stretched. Encouraging evidence came last year from a movie of epithelial cells recorded by Harvard University’s Matthew Gibson, Ankit Patel, Radhika Nagpal, and Norbert Perrimon. In the Harvard movie, newly formed cells stuck to and kept their immediate neighbors.
To find further supporting evidence, Hufnagel and Shraiman teamed up with Aurelio Teleman and Stephen Cohen of the European Molecular Biology Laboratory in Heidelberg, Germany. Teleman and Cohen coaxed disk cells into expressing a fluorescently tagged version of a morphogen called decapentaplegic (Dpp). Thanks to the fluorescence, Cohen and Teleman could monitor the Dpp concentration. As the disk grew, the concentration of Dpp at the peak fell, but the width of the profile remained the same. Although the result doesn’t prove the KITP model, it does rule out alternatives in which a changing profile regulates final size.
Teleman and Cohen could also artificially extend the Dpp profile by getting disk cells to express certain surface molecules that forestall Dpp’s degradation. The result of the experiment was to increase the disk’s final size, as expected.
Adjusting and measuring the force within a disk that lies inside a living maggot is extremely difficult. At present, computer simulation is the only way to see how force might influence size. Tinri Aegerter-Wilmsen of the University of Zürich and ETH Zürich in Switzerland performed a similar and independent simulation with colleagues from Zürich and the University of Konstanz in Germany. 3
The Zürich model contains the same physics as the KITP model but differs in one aspect. At the disk edge, the morphogen concentration is low and its gradient shallow. Peripheral cells, Aegerter-Wilmsen suspected, might fail to sense a growth-stopping threshold reliably. The Zürich–Konstanz model therefore features a stretching threshold instead. When the stretching threshold is exceeded, stretching can promote growth even without morphogen.
Morphogen-induced growth in the center stretches the periphery, which compresses the center. As the disk grows, stretched periphery widens and further compresses the center. Growth stops when compression shuts down cell division at the center and when the peripheral regions fall below the stretching threshold.
Bruce Edgar, a developmental biologist at the Fred Hutchinson Cancer Research Center in Seattle, Washington, sees the two models as essentially similar. “Growth suppression by compression is a plausible and attractive idea,” he says.
Although the underlying molecular mechanism hasn’t been identified, there are some candidates. When a disk cell encounters the morphogen wingless, a protein called β-catenin enters the cell’s nucleus to initiate the expression of proteins that forestall apoptosis and promote growth. Intriguingly, β-catenin has another role. The cytoskeletons of neighboring cells link to each other though membrane-spanning proteins called cadherins. The molecule that mediates the link is β-catenin.
Edgar points out another line of suggestive evidence. The genes Lethal giant disks, Disks large, and Scribble form a complex involved in cell adhesion. “If you knock them out,” he says, “the epithelium becomes a blob that never stops growing.”

As this simulated disk grows, tension at the periphery (top row, blue) and compression at the center (top row, red) both increase. Compression inhibits cell division, with the result that cell division (bottom row, red) occurs more or less evenly throughout the disk even though the morphogen profile (bottom row, green) is sharply peaked. In the KITP model shown here, a morphogen threshold regulates size. In the Zürich–Konstanz model, a stretching threshold regulates size.
(Adapted from ref. 2.)
References
1. B. I. Shraiman, Proc. Natl. Acad. Sci. USA 102, 3318 (2005).https://doi.org/PNASA6
2. L. Hufnagel, A. A. Teleman, H. Rouault, S. M. Cohen, B. I. Shraiman, Proc. Natl. Acad. Sci. USA 104, 3835 (2007).https://doi.org/PNASA6
3. T. Aegerter-Wilmsen, C. M. Aegerter, E. Hafen, K. Basler, Mech. Dev . (in press).




