Hybrid electrolysis produces record-high ammonia yields
The world population’s meteoric rise from 1.6 billion in 1900 to 7.7 billion today would not have been possible without the artificial synthesis of ammonia. The early-20th-century invention by Fritz Haber and Carl Bosch provides cheap and abundant fertilizer by fixing atmospheric nitrogen to a form that plants can use. But that success has not come without costs: The Haber–Bosch process requires high temperatures, high pressures, and molecular hydrogen, which, to date, comes from the catalytic conversion of fossil fuels such as natural gas.
Alternatives to Haber–Bosch use electricity and a catalyst to synthesize ammonia at ambient conditions with H2 from water, but those electrolysis experiments have had only limited success so far. Now, Ryan Hawtof, R. Mohan Sankaran
Joseph Toth, Case Western Reserve University
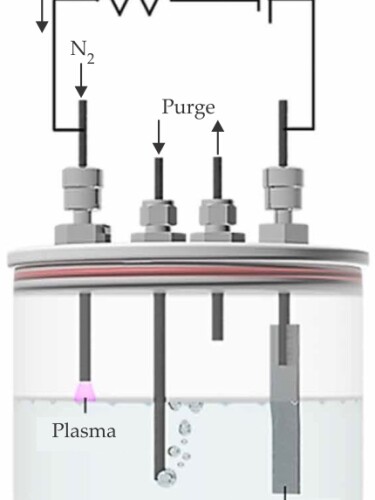
The design of the hybrid electrolysis system, shown in the schematic, replaces a traditional cathode with a plasma one (see the close-up photo below). “Plasmas contain charge and solutions contain charge, and it is natural to try to combine them,” says Sankaran. Merging plasmas and solutions with a platinum foil anode and a DC power supply, the researchers synthesized ammonia from N2 and H2 at ambient conditions; using a nonthermal plasma kept the system’s maximum temperature relatively low, at 65 °C. With the current tuned to 2 mA, the reaction proceeded at a production rate of 0.44 mg/hr. That may sound slow, but it’s up to 10 000 times faster than previous attempts.
“This is a major achievement,” says chemist Dennis Hetterscheid

Joseph Toth, Case Western Reserve University
The hybrid electrolysis method differs from past attempts in some important ways. The new technique takes advantage of free electrons dissolved in the water. Those solvated electrons, which the authors call “one of the most powerful reducing agents known,” form when N2 breaks down as it contacts the liquid-water surface. The solvated electrons are injected into the water by the plasma and can then generate hydrogen radicals to efficiently react with nitrogen and form ammonia.
Despite the promising yields and efficiencies, the hybrid electrolysis method probably won’t be replacing the Haber–Bosch process in the near future. “Making hydrogen radicals costs energy,” says Hetterscheid. “You cannot get this energy back at a later stage during the synthesis of ammonia.” Those costs, plus those that come with using a plasma cathode, are extensive: The new method uses about 200 times more energy than the Haber–Bosch process to produce the same amount of ammonia.

The production of ammonia is most efficient at a current tuned to 1–2 mA. More than 0.3 mg can be produced in 45 minutes.
Nevertheless, the new technique may have some utility. Unlike the Haber–Bosch process, the hybrid electrolysis method can operate at small scale and under ambient conditions using a DC power source, nitrogen gas, and water. “This would allow one to make ammonia anywhere in the world, even far from an existing Haber–Bosch and steam methane reforming plant,” says Sankaran. Fertilizer companies, for example, could make ammonia and form a distributed network of producers both to improve the security of supply and to lower distribution, storage, and transportation costs. And as fossil-fuel consumption decreases in the future, the new method could also be attractive for its renewable hydrogen source.
More about the authors
Alex Lopatka, alopatka@aip.org




