Dubna–Livermore Collaboration Forms Two New Superheavy Elements
DOI: 10.1063/1.1752412
Somewhere off the chart of known nuclides lies the last redoubt of nuclear existence: the long-sought island of stability. There, but for the stabilizing effect of filled shells of nucleons, a nucleus would be torn apart by the electrostatic force between protons.
Nuclear physicists want to reach the island to test their ideas of nuclear structure and stability. The island’s size and shape—even whether it’s really an island—are all uncertain. Nuclear chemists are also keen on gaining landfall. To characterize the chemistry of the periodic table’s ultimate members, they need isotopes stable enough to study.
The island’s rough position is known. Consensus has the last neutron shell filling up at 184 neutrons, but there’s less agreement about the protons. Early theoretical calculations put the last filled shell at 114; more recent ones suggest 120, even 126.
Making a superheavy nucleus of sufficient neutrons is perhaps the biggest barrier to landing on the island. Because the ratio N/Z of neutrons to protons in stable nuclei increases with Z, fusing any two stable nuclei together inevitably results in a nucleus of fewer than the ideal, stabilizing number of neutrons.
In 1999, a long-standing collaboration between the Joint Institute for Nuclear Research in Dubna, Russia, and Lawrence Livermore National Laboratory in California created several nuclei of 289114. Nine neutrons short of a full shell, the nuclei lasted a few seconds (see Physics Today, April 1999, page 21
If the Radioactive Isotope Accelerator is built, its intense neutron-rich beams should bring the island within reach. RIA shared third place in the US Department of Energy’s recent ranking of future facilities (see Physics Today, January 2004, page 23
Last summer, the Dubna–Livermore researchers, led by Dubna’s Yuri Oganessian, set out to create element 115. If, they reasoned, 114 protons fill a shell, then the alpha decay of element 115 across the filled shell to element 113 should reveal interesting, telltale effects.
To make element 115, the Dubna–Livermore team followed the same plan that produced elements 114 and 116. Using Dubna’s U400 cyclotron, the researchers bombarded a heavy-element target with ions of the neutron-rich isotope calcium-48. The 48Ca nuclide is stable, but its natural abundance, 0.2%, is so low that producing a usable quantity is extremely expensive. Dubna provided the 48Ca, while Livermore and Dubna each provided the target material, americium-243.
As one nears the island of stability, half-lives should lengthen. But that doesn’t mean fusing together two nuclei to make a new superheavy nucleus gets easier. In the highest reaches of the nuclide chart, yields barely top one a week. To catch such rarities, intense beams have to be kept running for at least a month; products have to be filtered without throwing away too many of the precious superheavies; and detectors have to spot the presence of the superheavy nuclei and trace their subsequent decays. Moreover, considering the retracted claim of element 118’s creation, the data analysis has to be irreproachable (see Physics Today, September 2002, page 15
Summer fruits
The experiment to make element 115 began at Dubna on 14 July and ran until 10 August. During that time, U400 hurled nearly 1019 48Ca ions at the 243Am target.
All but a small fraction of 48Ca ions pass through the thin target without fusing. Of the 48Ca ions that do fuse, most form nuclei that promptly undergo fission. The other fusion products, thanks to their forward momentum, join the host of surviving 48Ca ions and make their way to the Dubna Gas-Filled Recoil Separator.
Based on an idea from Albert Ghiorso, the separator consists of a large chamber filled with hydrogen. When colliding with H atoms, the fast 48Ca ions lose far more electrons than do the slow, heavy fusion products. The large powerful magnets that surround the chamber deflect the highly charged 48Ca ions so strongly that they hit the chamber’s sides before they exit. Livermore’s Ken Moody, who helped develop the prototype, estimates that only 1 in 1016 of the 48Ca ions escapes the separator, along with 1 in 106 of unwanted reaction products. But thanks to painstaking calibration, the separator lets 30–40% of the desired superheavy nuclei continue to the detector systems.
The first system the nuclei encounter after the separator is a pair of time-of-flight detectors that help screen valid events. After flying through these, the nuclei embed themselves in position-sensitive silicon detectors that convert a particle’s energy into electric charge.
Most of the superheavy nuclides discovered so far decay via a quick, short chain of alpha emissions followed by spontaneous fission. If that chain ends with a familiar nuclide, then, by adding two neutrons and two protons per alpha decay, one can count back to infer the superheavy’s original Z and N. But if, as was the case for element 115, the endpoint of the decay is a new, unfamiliar nuclide, then the counting back also needs a careful analysis involving semi-empirical predictions and comparisons with theory.
The deduction is possible because of the position sensitivity of the silicon detectors. Closely spaced events most likely originate in the same decaying nucleus. But ions and unwanted nuclei that sneak through the separator can masquerade as nearby decays. To reduce such undesirable coincidences, the Dubna-Livermore team turned off the 48Ca beam as soon as the TOF detectors logged a nucleus whose velocity matched that expected from a likely nucleus of element 115. Once the alpha decays ceased, the beam was turned back on.
The task of analyzing the events fell to the experiment’s two lead researchers, Dubna’s Vladimir Utyonkov and Livermore’s Joshua Patin. Although the two regularly communicate, they analyzed the putative events quite independently. Each has developed his own computer code—even the programming language they use is different (Utyonkov favors FORTRAN, Patin, C).
To the team’s relief, Utyonkov’s and Patin’s analyses agreed. In a month of beam time, the team created three nuclei of 288115, each of which alpha-decayed into 284113, and one nucleus of 287115, which alpha-decayed into 283113. The results, summarized by the figure on page 23, were published earlier this year. 1

Evidence for the creation of element 115 and its daughter 113 comes from four chains of alpha decays. The detected positions of successive events within each chain (green) were closely spaced. (But some individual decays, labeled “incomplete”, didn’t yield enough information for a definitive spatial measurement.)
(Adapted from ref. 1.)
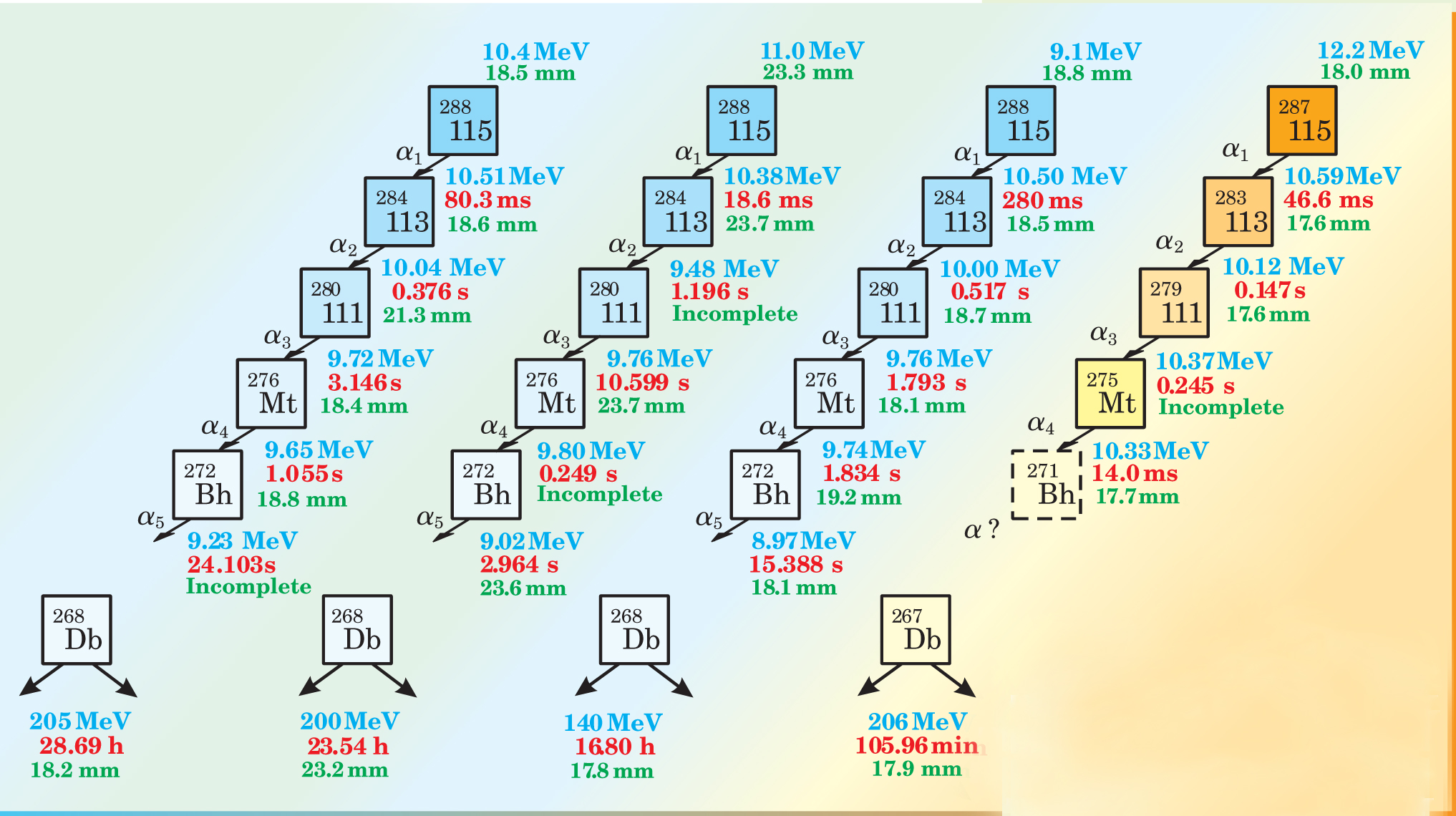
In the world of superheavy research, four decay chains amount to quite a catch. Unfortunately, the chains were too few to address the experimenters’ original aim of identifying a possibly filled proton shell.
Still, the experiment has helped to map part of the island of stability’s periphery. With lifetimes of 19–280 ms, the nuclei lasted longer than predicted—a hint, perhaps, that the island might be unexpectedly large.
References
1. Yu. Ts. Oganessian et al., Phys. Rev. C 69, 021601 (2004).https://doi.org/10.1103/PhysRevC.69.021601




