Diamond-defect NMR monitors a surface reaction
DOI: 10.1063/PT.3.4977
The study of surface chemistry has always involved a bit of a paradox. Chemical processes at solid–liquid and solid–gas interfaces are ubiquitous in batteries, industrial reactors, biomedical devices, and many other systems. But despite some research at moderate pressures (see the article by Gabor Somorjai and Jeong Young Park, Physics Today, October 2007, page 48
NMR spectroscopy is a time-honored tool for chemical analysis that works on bulk liquids, solids, and solid-like biomolecular systems. By measuring the precession frequency of spin-½ nuclei—for example, hydrogen-1, carbon-13, or fluorine-19—in a magnetic field, researchers can extract exquisite chemical information and even reaction dynamics. (See, for example, Physics Today, October 2019, page 21
An NMR measurement can be made vastly more sensitive by swapping the induction coils of a conventional apparatus for nitrogen–vacancy (NV) centers, a type of point defect in diamond that’s sensitive to magnetic fields. NV-center NMR has been under development for more than a decade, and although it hasn’t yet emerged as a laboratory mainstay, researchers have collected rudimentary spectra from samples a millionth the size of those needed for conventional NMR.
Now Kristina Liu, her PhD adviser Dominik Bucher, and their colleagues at the Technical University of Munich have demonstrated surface-sensitive NV-center NMR.
1
Their experimental setup, shown in figure
Figure 1.

No vacuum chambers are needed to study surface chemistry using diamond NV-center NMR. Here, Kristina Liu of the Technical University of Munich operates the relatively simple experiment, which uses green light from an inexpensive solid-state laser to read the NV centers’ spin states. The 2-mm-square diamond, not visible in the main image, is shown in the inset. (Photos by Andreas Heddergott, Technical University of Munich.)
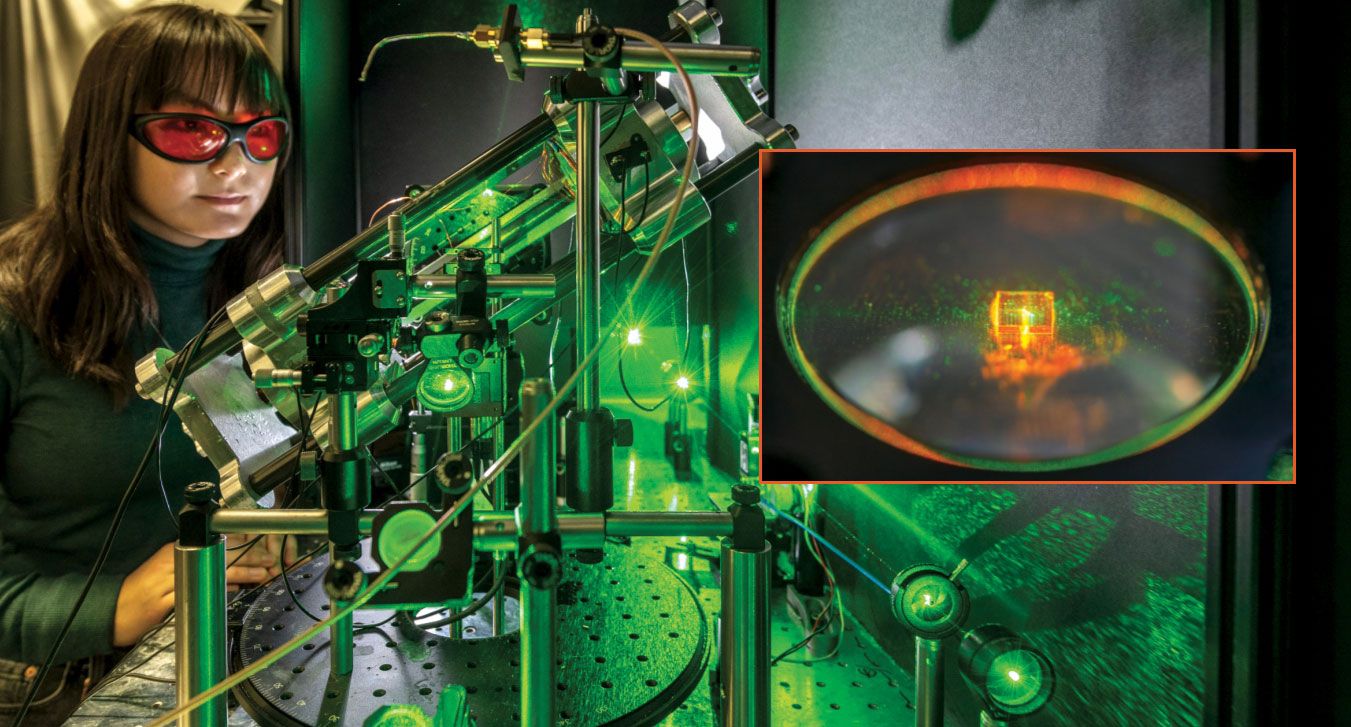
In related research, Peter Maurer (University of Chicago), Nathalie de Leon (Princeton University), and colleagues are working toward using NV centers to detect and study single proteins and other biomolecules. 2 They haven’t yet reached the stage of making NMR measurements themselves, but they’ve shown that with state-of-the-art biophysical techniques, they can tether biomolecules to NV-laden diamond without ruining either the biomolecular structure or the NV-center coherence. Combining their work with that of Bucher and colleagues could bring NV-center NMR into the realm of single-molecule biophysics.
Minuscule magnetometers
An NV center, as the name suggests, consists of a nitrogen atom and a vacancy at two adjacent sites in the diamond crystal lattice. The unpaired electrons bordering the vacancy form a spin-1 atom-like entity. Because the NV center is surrounded by an otherwise spinless sea of carbon, its spin is well shielded from its environment, and its quantum state retains its coherence as well as that of a trapped atom under vacuum. Moreover, NV-center spin states can be easily manipulated with microwave pulses and optically read with an inexpensive solid-state laser. The defects have been explored for applications in both quantum information and sensing. (See the article by Lilian Childress, Ronald Walsworth, and Mikhail Lukin, Physics Today, October 2014, page 38
Under the combined influence of a static magnetic field and a series of RF pulses, spin-½ nuclei in a sample precess, and the oscillating magnetic fields they generate affect the spin-state evolution of an NV center a few nanometers away. With a suitably chosen measurement on the NV center, researchers can identify the precession frequency, which gives them information about the precessing atom’s chemical identity and environment. That’s the basis for NV-center NMR.
In the first proof-of-principle experiments, researchers used NV centers to detect NMR signals from nearby 13C atoms in the diamond itself. Probing anything other than the inside of a diamond requires a careful balancing act: An NV center just below a diamond surface can pick up an NMR signal from a molecule just outside the diamond, but it may no longer fully benefit from the protective shielding of the carbon lattice.
The nature of the diamond surface, it turns out, matters a lot. When the dangling bonds at the edge of the lattice are capped with oxygen atoms, nearby NV centers retain their spin coherence, but when the surface is capped with hydrogen atoms, they don’t. Moreover, surface chemists are mostly interested in the chemistry of surfaces other than diamond: To use NV-center NMR for surface chemistry, it’s necessary to find a way to put an NV center in diamond in close enough proximity to the surface of some other solid—all without destroying the defects’ delicate spin states.
Bucher and colleagues and the Maurer–de Leon collaboration both identified the same solution: coating the diamond surface with a nanometer or two of aluminum oxide. The coating is easily done with atomic layer deposition (ALD)—although as Bucher points out, the experimental capabilities for ALD and NV-center NMR aren’t always present in the same lab. The diamonds, although synthetic, are expensive and in short supply (see Physics Today, March 2022, page 22
Both teams found that the ALD coating reduced the NV centers’ coherence time, but only a little, and the defects were still capable of a sensitive NMR readout. And because Al2O3 is commonly used as a support material in surface-science experiments, Bucher anticipates that it would be easy to coat with a second layer of yet another material to study the chemistry of almost any surface.
Surface sensitivity
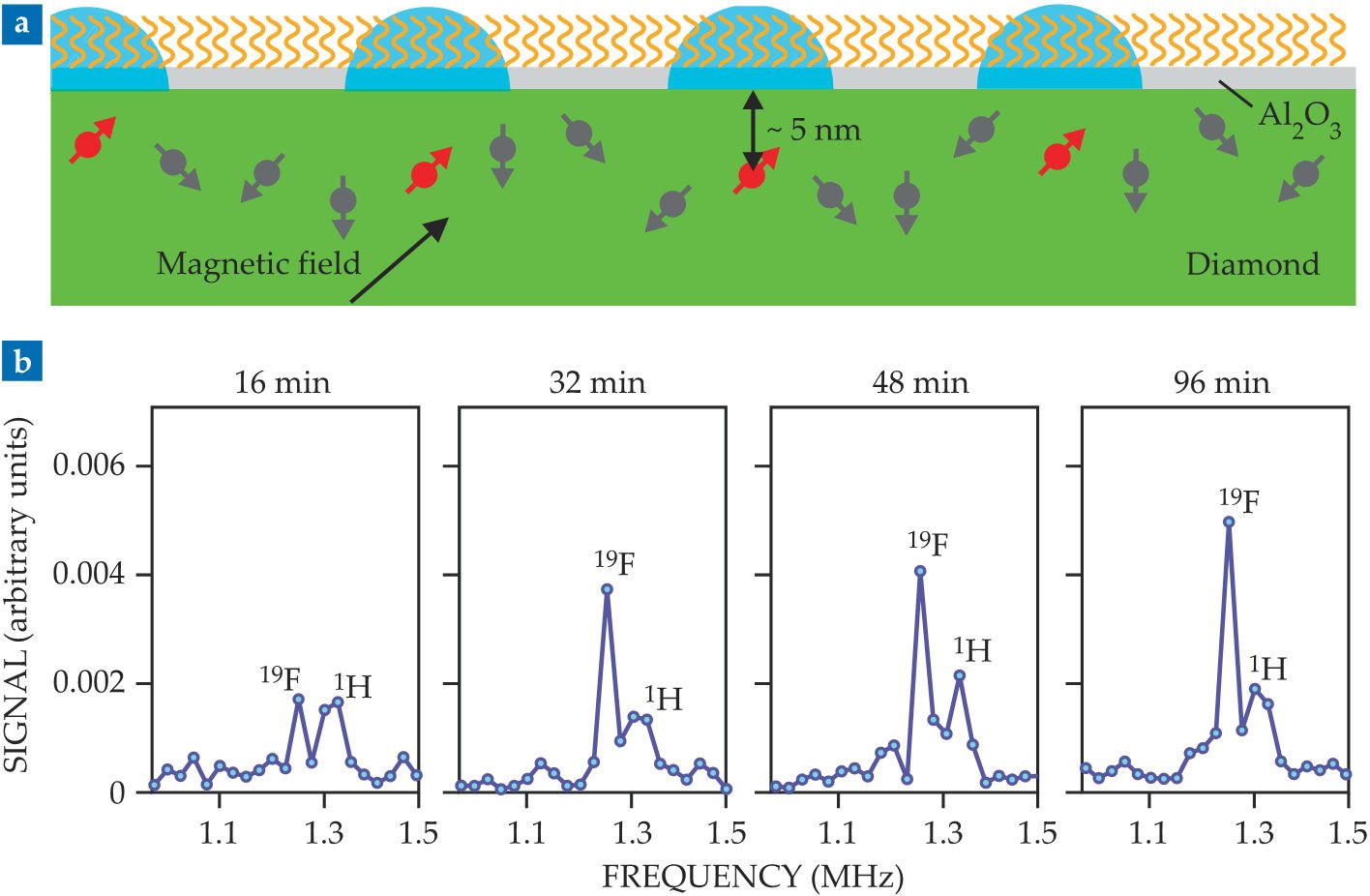
A schematic of the diamond sensors is shown in figure
Figure 2.
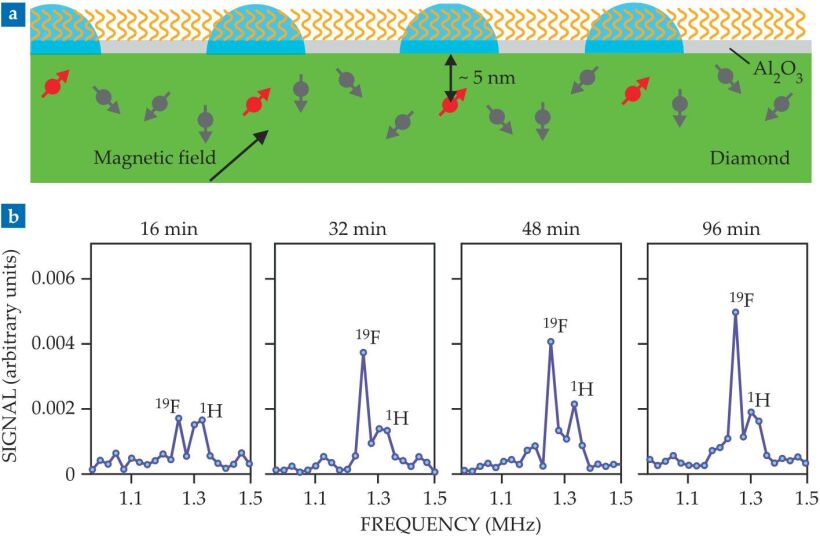
(a) A diamond chip (green) coated with a thin film of aluminum oxide (pale gray) forms the basis for NV-center surface NMR. NV centers aligned with the magnetic field (red) are sensitive to the adsorbed molecules (orange) in the detection volumes (blue) on the surface just above them. (b) Real-time NMR data follow the formation of a self-assembled monolayer of fluorinated molecules on the Al2O3 surface. The growing fluorine-19 peak indicates the molecules’ increasing surface coverage; the relatively constant hydrogen-1 peak comes from 1H atoms from an unconstrained source, probably in the diamond itself. (Adapted from ref.
For their work on biomolecules, Maurer and de Leon were interested not in studying the chemistry of the protein–surface interaction but in exploiting it to hold the proteins in place long enough to study them with NV-center NMR. In a bulk solution, proteins diffuse around randomly, and they spend little time in the NV centers’ detection volumes. A previous experiment on detecting single proteins with NV-center NMR immobilized the proteins by drying them onto the surface. 3 “That showed that the sensitivity is there,” says Maurer, “but the proteins were completely denatured, and their structure was destroyed. The next step is to do the same thing on an intact protein.”
Fortunately, biophysicists have developed a suite of chemical tools, drawing on a concept called click chemistry, for catching and holding biomolecules. A click reaction involves a pair of chemical groups that quickly “click,” or bind together, whenever they’re in close proximity. By placing one group on a biomolecule of interest and the other on a substrate, researchers can reliably join the biomolecule to the substrate.
Maurer, de Leon, and colleagues showed that they could attach half of a click-chemistry pair to an Al2O3-coated NV-laden diamond and use it to immobilize biomolecules on the surface. Using optical fluorescence, they showed that the surface-bound biomolecules retained their structure for several days—a promising step toward studying the molecules with NV-center NMR.
Bucher and colleagues, for their proof-of-principle surface-chemistry experiments, used fluorine-rich molecules and focused on detecting their 19F signals, rather than the 1H (proton) signals more typical of conventional NMR. “We’re avoiding studying protons for now,” says Bucher, “because protons are everywhere—even in the diamond—and it’s not well controlled where the signal is coming from.”
For example, when the researchers monitored the formation of a self-assembled monolayer on the Al2O3 surface, they saw a relatively unchanging 1H peak, as shown in figure
Regaining resolution
Collection of real-time data under chemically relevant conditions is a step forward for NV-center NMR, but Bucher and colleagues’ spectral resolution is in a sense a step backward. In conventional NMR (and even some previous NV-center NMR experiments; see Physics Today, May 2018, page 21
Those differences, called chemical shifts, convey highly detailed information about chemical structure, but they’re unresolved in the new work. Says Bucher, “I know chemists are going to look at this and ask, ‘Where’s my chemical information?’”
Such poor resolution is a known issue for NMR on a solid or solid-like system. In an isotropic medium, such as a liquid, molecules tumble around rapidly, and the effect of their orientation with respect to the applied magnetic field is averaged out. But in an anisotropic environment, such as a solid or interface, molecular orientations are frozen in place, and the slight differences in precession frequency from molecule to molecule smear out the spectral lines into an unresolved blob.
There are ways of regaining the resolution in solid-state NMR. For example, spinning the sample at a so-called magic angle with respect to the magnetic field re-creates some of the effect of isotropic liquid tumbling. (See the article by Clare Grey and Robert Tycko, Physics Today, September 2009, page 44.
Instead, Bucher seeks to look beyond spin-1⁄2 nuclei to quadrupolar isotopes with spin 1 or greater. In nuclear quadrupole resonance, or NQR, the precession frequencies are primarily determined not by interaction with an external field but by the electric field gradient produced by the surrounding atoms. That is, molecular orientation with respect to the field doesn’t matter as much, and an applied field may not even be necessary. (See the article by Micah Ledbetter and Dmitry Budker, Physics Today, April 2013, page 44
NV-center NQR, therefore, could convey detailed chemical information about a surface without the technical challenges of solid-state NMR. “These are very new ideas,” says Bucher, “but I think that is the future of this technology.”
References
1. K. S. Liu et al., Proc. Natl. Acad. Sci. USA 119, e2111607119 (2022). https://doi.org/10.1073/pnas.2111607119
2. M. Xie et al., Proc. Natl. Acad. Sci. USA 119, e2114186119 (2022). https://doi.org/10.1073/pnas.2114186119
3. I. Lovchinsky et al., Science 351, 836 (2016). https://doi.org/10.1126/science.aad8022
More about the authors
Johanna L. Miller, jmiller@aip.org




