A triatomic molecule is laser cooled and trapped
DOI: 10.1063/PT.3.5057
In March 2020, as the world was grappling with the implications of the COVID-19 pandemic, John Doyle and colleagues at Harvard University were faced with the bleak prospect of shutting down their experiments on ultracold atoms and molecules, perhaps indefinitely. So they turned their physics expertise to a rather different but more timely set of problems: Could N95 masks, in perilously short supply at the time, be decontaminated and reused? 1 And how could the risks of airborne disease transmission be most effectively mitigated in a laboratory or office setting? 2
Through their work, they helped Harvard develop a plan to safely and quickly reopen its research labs—including their own, shown in figure
Figure 1.

A thicket of optical elements directs the laser beams that cool and trap the triatomic molecule calcium monohydroxide. Laser cooling and magneto-optical trapping of atoms is standard practice, and a few groups have succeeded in cooling and trapping diatomic molecules. But never before has a three-atom molecule received the same treatment. (Photo by Loïc Anderegg.)
That opportunity has paid off. In one of a steady stream of papers published since the start of the pandemic, they’ve now demonstrated the laser cooling and magneto-optical trapping of calcium monohydroxide (CaOH), the first three-atom molecule to be so cooled and trapped. 3 The extension of ultracold techniques to larger molecules promises to make possible new experiments in quantum information, tests of fundamental physics, and more.
Will the cycle be unbroken?
Laser cooling and trapping of atoms is a decades-old technology that garnered the 1997 Nobel Prize (see Physics Today, December 1997, page 17
The success of that method hinges on getting the atom to reliably return to its ground state. If there’s some other low-lying state that it can relax into instead, researchers need to add another laser to repump the atom from that state; if the atom ever ends up in a so-called dark state that isn’t repumped, the cooling cycle ends and the atom is lost from the experiment. The easiest atoms to laser cool are therefore those with the simplest energy-level structures, mostly alkali metals such as potassium and rubidium and alkaline earth metals such as calcium and strontium.
Extending ultracold methods from atoms to molecules is useful for a wide range of experiments. Some of those experiments are obvious, such as studying chemical reactions in the quantum regime (see the article by Debbie Jin and Jun Ye, Physics Today, May 2011, page 27
But all the challenges of laser cooling atoms are compounded in molecules, which possess not only electronic quantum states but also quantized rotations and bond vibrations. The veritable continuum of low-lying states would, in the general case, require many dozens of repumping lasers to keep under control.
Because of the difficulty of cooling molecules directly, most ultracold-molecule researchers build their molecules from atoms that are already cooled. Although that approach works well, it yields exotic, weakly bound molecules such as KRb (see Physics Today, February 2020, page 12
But some special molecules can be cooled directly without too much trouble. The trick is to find a molecule whose ground and excited electronic states have nearly the same equilibrium shape. (For diatomic molecules, the “shape” is merely the length.) The molecule can be cycled between those states without stretching it, and most molecules reliably relax to the vibrational ground state. Occasionally they might end up with one or a few quanta of vibrational energy, but a small number of repumping lasers can coax them back to the vibrationless state.
Two molecules that fit the bill are strontium monofluoride and calcium monofluoride. In each case, the alkaline earth metal atom—Sr or Ca—has an extra electron that doesn’t participate in the metal–fluorine bond. Exciting that electron doesn’t change the bond length much at all. Both molecules can be laser cooled with just a handful of additional repumping lasers. (See Physics Today, January 2010, page 9
Importantly, there are multiple tiers of what’s meant by “laser cooling.” Because of their extra electrons, SrF and CaF aren’t stable enough to be carried around in bottles; they have to be synthesized from their constituent atoms in a molecular beam. It’s relatively straightforward to apply lasers perpendicular to the molecular beam to cool the molecules’ transverse motion while they hurtle along at some 100 m/s—and for some applications, that form of cooling is enough.
For experiments that require lower temperatures or optical lattices, however, researchers need to catch the molecules in a trap, which requires first slowing the molecular beam to a virtual stop. To do so with just lasers, they need to get the molecules to cycle more than 10 000 photons without ever landing in a dark state. That challenging feat, even for diatomic molecules, has been achieved by only a handful of groups in the world so far.
The bends
Going from two atoms to three introduces another important degree of freedom: Not only can triatomic molecules stretch and rotate, but they can also bend. The bending mode gives rise to some potentially interesting new effects. For example, in strontium monohydroxide, it so happens that three quanta of bending have almost exactly the same energy as two quanta of the Sr–O stretch. That accidental degeneracy makes the SrOH spectrum potentially sensitive to some postulated types of dark matter.
For another example, exciting the bending mode breaks an otherwise linear molecule’s linear symmetry. A bending molecule therefore has quantized rotational motion not just from tumbling end over end but also from turning like a spindle about the molecule’s length. For complicated quantum mechanical reasons, molecules that possess angular momentum about their internuclear axes are extremely easy to align with an applied field. That capability comes in handy in searches for the electron electric dipole moment, use of the molecules as qubits, and more.
But the bending mode introduces the same challenge to laser cooling as a diatomic molecule’s stretching mode does: It creates more vibrational states into which the molecule might relax and break the optical-pumping cycle. Fortunately, the same advantage of SrF and CaF also applies to CaOH (which Doyle and colleagues chose over SrOH simply because its transitions lie at more convenient laser frequencies): The Ca atom’s extra electron doesn’t participate in bonding, so most molecules relax into the vibrational ground state. But a few relax into states excited in the Ca–O stretch, the bend, or both.
Doyle and colleagues transversely cooled CaOH in a molecular beam just before the start of the pandemic. It took six lasers, one to drive the main transition and five to repump vibrationally excited states.
4
That scheme cycled an average of about 1000 photons per molecule. To reach the 10 000-photon threshold required to slow the beam to a stop, the researchers had to add six more laser frequencies, for a total of 12, as depicted in figure
Figure 2.

Twelve lasers combine to laser cool calcium monohydroxide. (a) The 626 nm laser cycles the molecule between the ground and electronically excited states, and 11 more lasers repump molecules that relax into vibrationally excited states. (b) The blue circles show the cumulative number of photons cycled as each new repumping laser is added. A six-laser scheme (from “ground state” through “bend 3”) cycles just 1000 photons per molecule. But using all 12 lasers increases that number to 12 000—enough to slow and trap the molecular beam. (Adapted from ref.
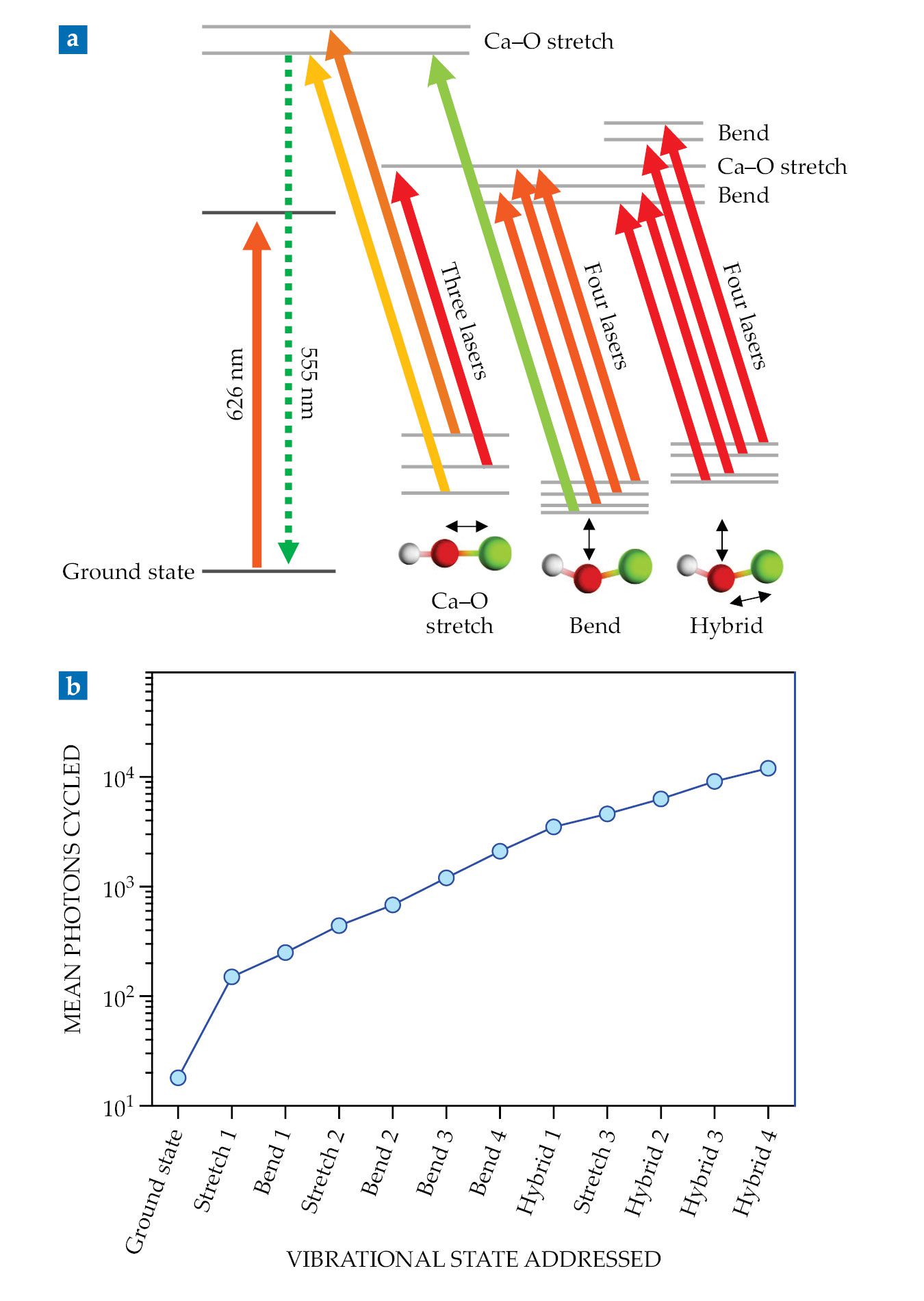
Fortunately, the molecules don’t all fall into a dark state after the same number of cycles. The mean number of photons cycled, plotted in figure
Bigger and bigger
Bending and Ca–O stretching aren’t the only vibrational modes available to CaOH. There’s also stretching of the OH bond, which the Harvard researchers’ laser scheme doesn’t address at all. But that’s because it didn’t need to: Optically cycling CaOH molecules almost never end up with any quanta of O–H stretching motion. It’s intuitively easy to understand why. The cycling excitation is centered on the Ca atom, which is physically separated from the OH bond. The two parts of the molecule simply have little to do with each other.
That line of reasoning would seem to imply that laser-coolable molecules can be made arbitrarily large: Just attach a Ca (or Sr) atom to an O atom to just about anything. As with CaOH, one could optically excite the unpaired electron, repump a few stretching and bending vibrational states, and leave the rest of the molecule alone.
The reality is almost certainly not so simple. Larger molecules have greater mass, so they’d have to cycle more photons to be slowed to a stop. And the sheer density of states of large molecules can introduce complicating effects, such as the coupling of disparate vibrational modes of similar energy.
Nevertheless, preliminary research suggests that the outlook for laser cooling larger molecules is good. Theory groups led by Anna Krylov, Svetlana Kotochigova, and Anastassia Alexandrova have analyzed the vibrational structures of many candidate molecules, and they predict that coolable molecules can incorporate some rather large organic structures, including benzene rings. 5 On the experimental side, Doyle and colleagues have already done transverse in-beam cooling of calcium monomethoxide (CaOCH3), although they still have a long way to go before they can catch that molecule in a trap. 6
Cooling larger molecules would introduce even more richness to cold-molecule experiments. For example, if researchers could cool a molecule that breaks mirror symmetry, they could investigate whether the laws of physics treat such asymmetric molecules differently from their mirror-image counterparts. More broadly, by bringing all the well-known power of chemistry to bear on the ultracold regime, researchers could design ultracold molecules with shapes and properties to suit any purpose. “We don’t know whether our techniques are as broadly applicable as that,” says Doyle. “That’s a frontier scientific question. We’re very interested in seeing what’s possible.”
References
1. L. Anderegg et al., PLoS One 15, e0234851 (2020). https://doi.org/10.1371/journal.pone.0234851
2. B. L. Augenbraun et al., J. Occup. Environ. Hyg. 17, 447 (2020). https://doi.org/10.1080/15459624.2020.1805117
3. N. B. Vilas et al., Nature 606, 70 (2022). https://doi.org/10.1038/s41586-022-04620-5
4. L. Baum et al., Phys. Rev. Lett. 124, 133201 (2020). https://doi.org/10.1103/PhysRevLett.124.133201
5. J. Kłos, S. Kotochigova, Phys. Rev. Res. 2, 013384 (2020); https://doi.org/10.1103/PhysRevResearch.2.013384
M. V. Ivanov et al., J. Phys. Chem. Lett. 11, 6670 (2020); https://doi.org/10.1021/acs.jpclett.0c01960
C. E. Dickerson et al., Phys. Rev. Lett. 126, 123002 (2021). https://doi.org/10.1103/PhysRevLett.126.1230026. D. Mitra et al., Science 369, 1366 (2020). https://doi.org/10.1126/science.abc5357
More about the authors
Johanna L. Miller, jmiller@aip.org




