A single-component organic crystal is ferroelectric at room temperature
DOI: 10.1063/1.3397032
Plastics and other organic materials can be fashioned into bendy, stretchy sheets. Exploiting that flexibility for electronic devices entails finding organics that exhibit useful phenomena. Display panels in cell phones already make use of the semiconductivity and light emission of two organics, polyfluorene and poly(phenylene-vinylene). Ultrathin e-readers are already on the market.
Now, Sachio Horiuchi of Japan’s National Institute of Advanced Industrial Science and Technology, Yoshinori Tokura of the University of Tokyo, and their collaborators have found an organic material that exhibits another technologically useful phenomenon, ferroelectricity: the ability to switch polarization in response to an electric field. 1 Ferroelectrics, as well as their cousins, piezoelectrics and pyroelectrics, have a host of applications, including nonvolatile memory, mechanical actuation, and temperature sensing.
The new ferroelectric is the crystalline form of croconic acid, an organic dye first synthesized in 1825 by Heinrich Gmelin. He named his discovery after κρóκoς, the Greek word for saffron. In addition to its industrial uses as a dye, croconic acid is an attractive building block for supramolecular chemistry, the bottom-up self-assembly of nanostructured materials.
Figure 1 shows the structural origin of that attraction and of the solid’s ferroelectricity. Each croconic acid molecule consists of a pentagonal ring of carbon atoms bound to oxygen and hydroxyl (OH) groups. The carbon atoms’ p orbitals overlap and merge to form π orbitals above and below the ring. The merging weakens the O-H bonds, thereby loosening the hydrogen atoms. In water, the molecules dissolve into C5O5 2- anions and H+ cations—hence the acidity. For supramolecular chemists, the anions’ five symmetric, outwardly pointing binding sites turn the anions into tiles that can be assembled into two-dimensional sheets.

Figure 1. Croconic acid forms crystals of stacked puckered sheets of hydrogen-bonded molecules. (a) Two configurations of hydrogen bond are present, shown here in green and blue. (b) Because of the shape of the molecules and the arrangement of the intermolecular bonds, croconic acid has a spontaneous polarization ±P S. Applying an electric field in the right direction can reverse the polarization. (c) The field also changes the bond configuration of each molecule. In some inorganic ferroelectrics, the entire crystal structure breaks symmetry. In croconic acid, only the shuttling H atoms break symmetry.
(Adapted from ref. 1.)
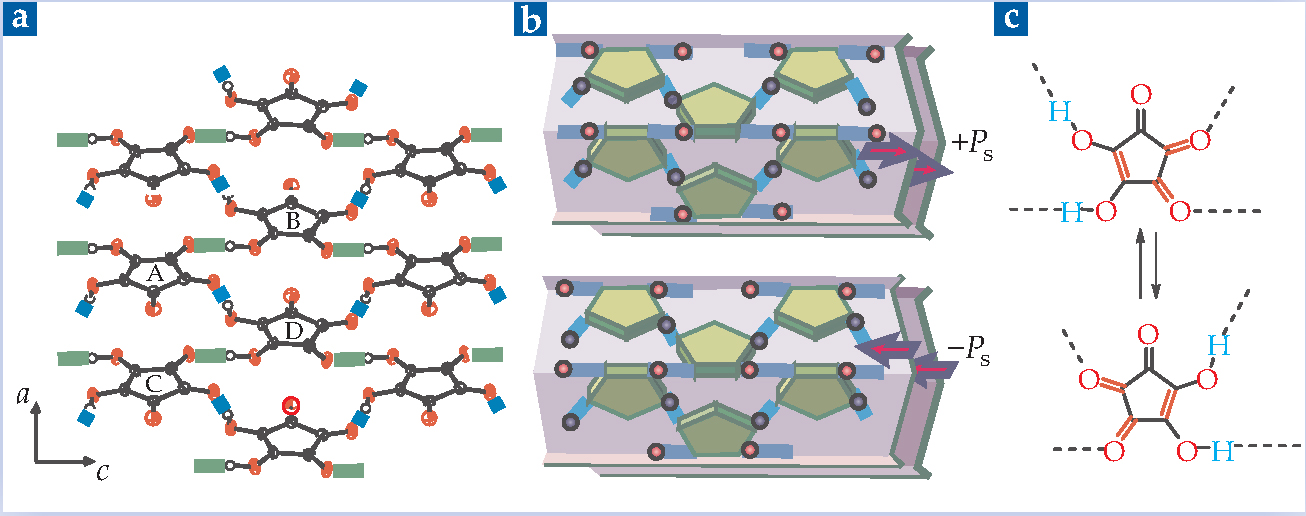
In a croconic acid crystal, the loosened H atoms form a network of hydrogen bonds that holds the crystal together. Two orientations of hydrogen bond are present, indicated in figure
Properties
Despite the straightforward origin of its ferroelectricity, croconic acid was not initially the target of Horiuchi and Tokura’s search for organic ferroelectrics. The researchers were exploring a general class of potential ferroelectrics that depend on the shuttling of H atoms between acids and bases. Croconic acid was one of the candidate acids. To learn more about it, they looked up its structure, which had been determined nine years ago by Dario Braga, Lucia Maini, and Fabrizia Grepioni of the University of Bologna in Italy.
The structure suggested that croconic acid might be ferroelectric by itself. Indeed, once Horiuchi, Tokura, and their collaborators had purified and crystallized the compound, they duly found the hysteresis loops of polarization versus electric field that characterize ferroelectrics. Figure 2 shows some examples.
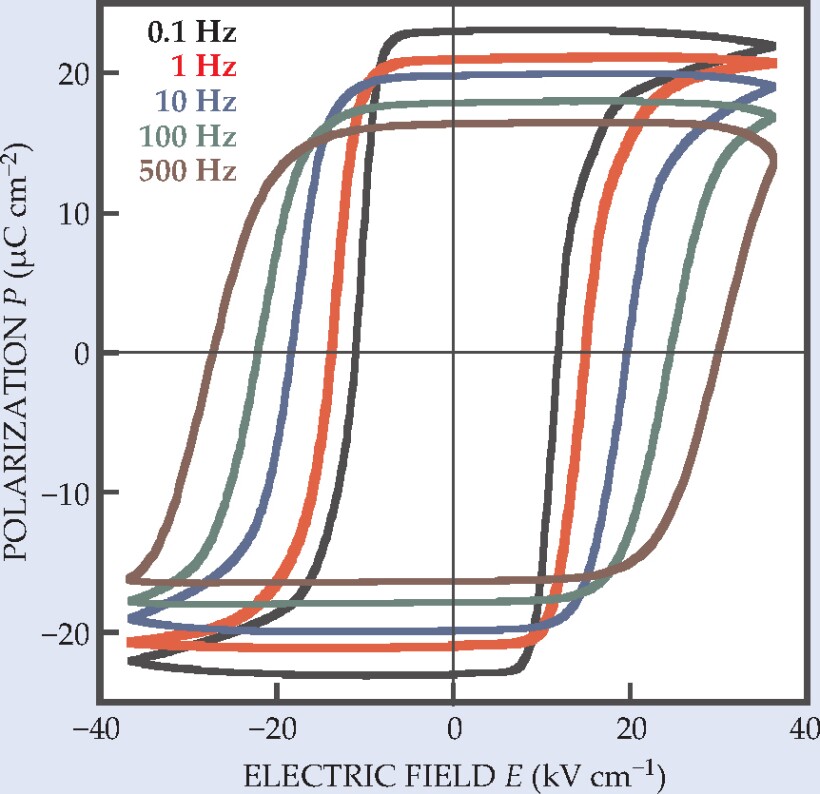
Figure 2. Hysteresis loops derived for a crystal of croconic acid at five different frequencies of an AC electric field with a triangular (zigzag) waveform. The maximum polarization amplitude at 0.1 Hz is among the highest of any organic ferroelectric.
(Adapted from ref. 1.)
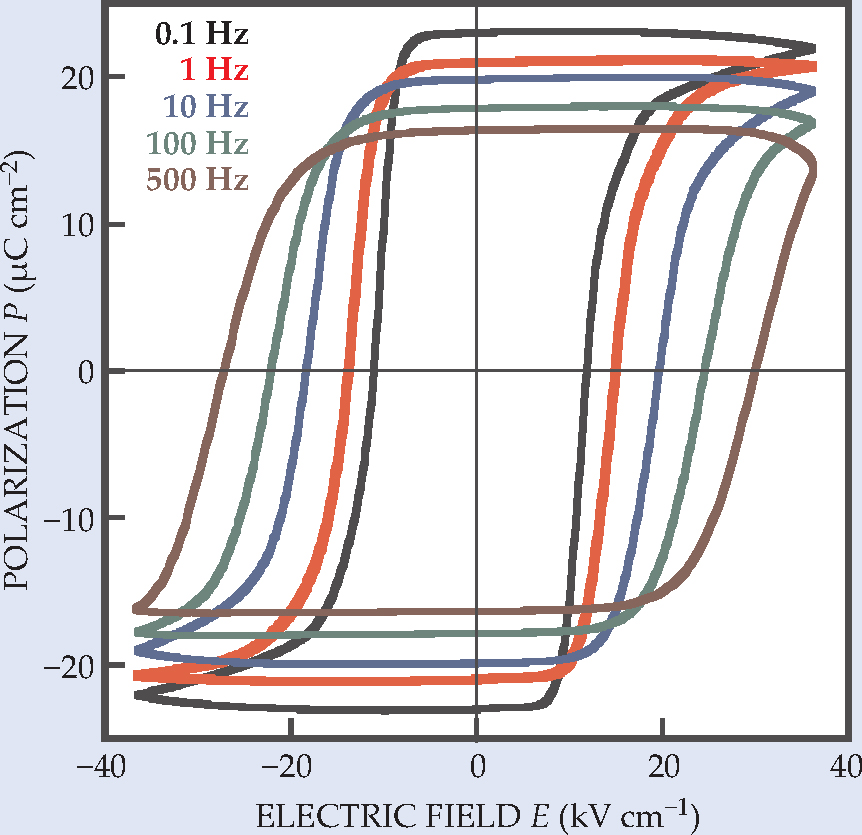
Several aspects of croconic acid’s ferroelectricity are noteworthy. At 21 µC cm-2, the polarization of croconic acid is the highest of all organic ferroelectrics and is comparable to that of barium titanate, an inorganic piezoelectric used in microphones, loudspeakers, and other transducers. Croconic acid’s switching field of around 10 kV cm-1 is also comparable to that of barium titanate; its hysteresis loops approach the rectangular ideal except at high switching frequency, when incipient electrical conduction rounds, tilts, and flattens them.
Another intriguing property is the critical temperature T c at which thermal fluctuations overwhelm an electric field’s ability to align the polarization. It turns out that croconic acid crystals start to decompose at 150 °C before T c is reached. According to Horiuchi and Tokura, croconic acid is the first example of both high spontaneous polarization and room temperature operation among single-component low-molecular-mass organic ferroelectrics. All previously discovered organic ferroelectrics are acid-base binaries.
To verify that hydrogen bonding is indeed responsible for the ferroelectricity, it’s important to determine the distance between the H atoms of one molecule and the O atoms of its putatively hydrogen-bonded neighbors. A direct measure of that distance with the most convenient method, x-ray diffraction, is difficult because x rays barely scatter off the H atoms’ tiny electron clouds. The researchers could, however, measure the intermolecular oxygen distance. At 2.62-2.63 Å, the derived value implies the hydrogen bonding is moderate: strong enough to bind the molecules together, but weak enough to allow the H atoms to move.
It’s also important to confirm that the off-center positioning of the H atoms is energetically favored. Horiuchi and Tokura’s collaborators, Gianluca Giovannetti and Silvia Picozzi of the University of L’Aquila in Italy, obtained the confirmation using density functional theory. Their calculations also reproduced the measured value of the polarization.
In another experiment, Horiuchi and Tokura looked for an optical property of ferroelectric crystals: frequency doubling. Because of a ferroelectric’s symmetry, light of frequency ω can cause the polarized charge distribution to oscillate and generate light of frequency 2ω, provided the crystals, or more precisely, their ferroelectric domains, are favorably aligned. By sweeping a narrow laser beam over a sample, the researchers could map croconic acid’s domain walls. Pristine crystals showed weak, patchy 2ω emission. Applying an electric field aligned the domains, turning almost the entire surface of the crystal into a bright 2ω emitter.
Despite its impressive performance, croconic acid is not likely to become an industrially useful ferroelectric, at least not its pure, unmodified form. For one thing, the crystals appear to deteriorate after a few months under ambient conditions. Another drawback is that croconic acid’s ferroelectricity occurs in its brittle, crystalline state. If the compound is to help fulfill the promise of thin, flexible electronics, a way has to be found to make it into a polymer while retaining enough of its ferroelectricity to be useful. Croconic acid’s significance is likely to serve as a structural paradigm that could be exploited in other materials, either from the same branch of the family tree or from new ones.
References
1. S. Horiuchi et al., Nature 463, 789 (2010). https://doi.org/10.1038/nature08731




