X-ray diffraction details water’s path through a cell pore
DOI: 10.1063/PT.3.2068
Certain biological processes—producing tears and processing urine, for instance—require cells to expel or take in water faster than it can diffuse through a cell membrane. In those cases, cells deploy aquaporins, special proteins that straddle a cell membrane and form water-permeable pores. Discovered two decades ago by Peter Agre (see Physics Today, December 2003, page 27
The proteins are highly selective gatekeepers. All of the dozens of known variants contain a narrow passageway—a so-called selectivity filter (SF), positioned near the pore’s extracellular opening—that stems the flow of large solutes. Likewise, strategically positioned charge centers embedded in the pore wall create potential barriers that block the flow of ions.
Harder to explain, however, is aquaporins’ impermeability to protons. Because protons can hop freely along a network of hydrogen-bonded water molecules, one would expect a water-filled pore to conduct protons in much the same way that a wire conducts electrons—and that would make it impossible for a cell to preserve the transmembrane potentials needed to power cellular machinery.
Presumably, aquaporins possess some structural feature that precludes the formation of a pore-spanning hydrogen-bond network. In one theory, now more than a decade old, the key feature is a positively charged NPA constriction—so called because it’s lined with asparagine (N), proline (P), and alanine (A) amino acids. Located at the pore’s midpoint, the NPA constriction generates an electric field thought to configure nearby water molecules so that their oxygen atoms face one another—an orientation ill suited to hydrogen-bond formation.
Starting around 2006, a series of experiments by Eric Beitz (University of Kiel, Germany) and collaborators began to cast doubt on that prevailing thinking. 1 The researchers generated mutant aquaporins having a charge-neutral NPA constriction, and they found that those pores showed negligible change in their proton permeability. When they instead modified amino acids in the SF, protons began to seep through.
Now a collaboration led by Richard Neutze (University of Gothenburg, Sweden) and Emad Tajkhorshid (University of Illinois at Urbana-Champaign) has used x-ray crystallography to construct the most detailed picture to date of the inner workings of an aquaporin protein. 2 And that picture seems to confirm at least a partial role for the SF in aquaporin’s proton blockade.
Bond hunting
Membrane proteins are notoriously difficult to crystallize and typically yield x-ray structures with resolutions of no better than 2 Å or so. Working with a yeast aquaporin that proved exceptionally amenable to crystallization, however, Gerhard Fischer, a graduate student in Neutze’s lab, was able to grow crystals large enough and uniform enough to diffract at 0.88-Å resolution—a record for a membrane protein.
The diffraction data yielded a detailed electron-density map of the inner region of the pore, as shown in the figure below. Gray mesh contours indicate electron clouds of the protein’s atoms; orange contours indicate electron clouds of water molecules occupying energetically favorable sites inside the pore. During transport, water molecules traverse the pore in single file by hopping from one site to the next.

An electron-density map of a yeast aquaporin shows two structural motifs—the selectivity filter and the so-called NPA constriction—thought to factor into the pore’s impermeability to protons. Gray mesh contours show the electron clouds of the protein’s carbon (gray), nitrogen (blue), oxygen (red), and sulfur (yellow) atoms; orange contours show the electron clouds of water molecules inside the pore. In the inset, a so-called hydrogen omit map of the selectivity filter, green contours correspond to hydrogen atoms on the protein. The atoms’ arrangement suggests multiple water–pore hydrogen bonds, including the two indicated here by the dashed lines. (Adapted from ref.
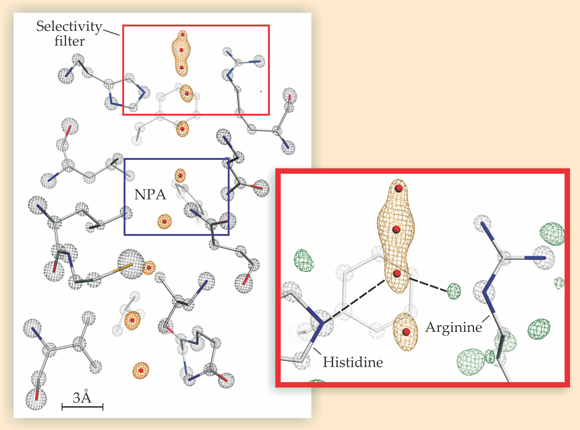
To reconstruct a hydrogen-bond network, one needs to know which so-called donor atoms (always hydrogen) interact with which acceptors (oxygen, nitrogen, or some other highly electronegative atom). But hydrogen atoms—whether in the protein or in water—lack sufficient electron density to produce distinct x-ray diffraction peaks and therefore don’t appear in the electron-density map. To clearly see them, Neutze and coworkers constructed what’s known as a hydrogen omit map, a map of the difference between the system’s measured electron density and the expected electron density if the hydrogen atoms were removed. In such a map, hydrogen atoms appear as regions of excess measured density. In the inset, excess density corresponding to the protein’s hydrogen atoms is shown in green. (Water’s hydrogen atoms aren’t visible.)
Omit maps aren’t new to protein crystallography, but Neutze and coworkers are the first to generate one detailed enough to see an aquaporin’s hydrogen atoms individually. That allowed the researchers to determine which atoms embedded in the pore wall were potential hydrogen-bond acceptors and which were potential donors. The detected structural arrangement of those acceptors and donors suggests that water molecules in the SF are likely to form hydrogen bonds with atoms in the pore wall rather than with one other.
The inset, for example, indicates two hydrogen bonds lying between a water molecule in the SF and sites on histidine and arginine amino acids that line the pore wall. A third bond between the molecule and the pore lies normal to the page and isn’t shown. Because water can form at most four hydrogen bonds, the molecule can’t participate in enough water–water bonds to propagate a chain. Other water molecules in the SF are similarly tied up by the pore, and the result is a fragmented hydrogen-bond network. Comments Beitz, “This will likely bring to an end a 10-year discussion in the aquaporin community. It’s now quite clear that both the NPA constriction and the SF contribute to blocking protons.”
The team’s x-ray structure also yielded a surprise discovery: The four water sites in the SF are spaced so closely—roughly 1.5 Å, about 1 Å less than the preferred water-molecule spacing—that no two neighboring sites can be occupied simultaneously. Molecular dynamics simulations by collaborators Tajkhorshid and Giray Enkavi show that pairs of water molecules move between those sites in highly coordinated fashion: Molecules in the first and third positions jump in unison to the second and fourth positions. The finding points to a possible evolutionary link with potassium ion channels, which convey potassium ions in similar fashion.
References
1. E. Beitz et al., Proc. Natl. Acad. Sci. USA 103, 269 (2006); https://doi.org/10.1073/pnas.0507225103
B. Wu et al., EMBO J. 28, 2188 (2009). https://doi.org/10.1038/emboj.2009.1822. U. K. Eriksson et al., Science 340, 1346 (2013). https://doi.org/10.1126/science.1234306




