Two experiments, two takes on electric bacteria
DOI: 10.1063/1.3529396
Metabolism, the collection of biochemical reactions that convert nutrients into energy, comes with a catch: It leaves behind a bevy of unneeded electrons, which the cell must somehow rid itself of. That, in essence, is why we breathe—the oxygen molecules we inhale get absorbed in the bloodstream and taken up by our cells to act as terminal electron acceptors in the metabolic process, ultimately emerging in the form of water.
But some harsh environments don’t afford the luxury of soluble, ingestible electron acceptors. For those cases, microbes such as the marine bacterium Shewanella oneidensis have evolved a unique contingency plan: If the acceptor can’t get to the electrons, they send the electrons to the acceptor. Shewanella, for instance, can export electrons to extracellular minerals such as iron, manganese, and uranium oxides and produce a current that researchers are now learning to harness in microbial fuel cells and other applications. Exactly how the microbes pull off the feat, however, remains something of a mystery.
Two potential explanations have gained traction: Either the microbes secrete shuttle molecules that diffuse to a metal surface, deposit electrons, and then return to start the process anew; or, on contacting a suitable metal, they simply pass electrons directly across their cell membrane. In the past decade, evidence has accumulated on both sides of the debate. On one hand, several studies point to riboflavin—commonly known as vitamin B2 — and other flavin molecules as Shewanella’s crucial shuttles. On the other hand, most researchers agree that some electricity-producing microbes, such as Geobacter, are incapable of secreting such shuttles. If those microbes transfer electrons via direct contact, as appears to be the case, it’s plausible that Shewanella, a fellow member of the phylum proteobacteria, could do the same.
New studies from two groups—one headed by Charles Lieber of Harvard University and Bradley Ringeisen of the US Naval Research Laboratory in Washington, DC; the other by Mohamed El-Naggar of the University of Southern California and Yuri Gorby of the J. Craig Venter Institute in San Diego, California—address the question via decidedly different approaches. Their contrasting results suggest there may not be a simple answer.
Limited access
Lieber and Ringeisen set out to investigate Shewanella’s electron transfer with a miniature fuel-cell experiment. 1 They fashioned an array of gold-titanium composite nanoelectrodes on a glass chip, to which a microbial culture would be exposed. The researchers’ trick, then, was to carefully control how microbes accessed the nanoelectrodes. To that end, they covered the nanoelectrode array with a 400-nm-thick layer of insulating silicon nitride. They then etched through the insulating layer to expose alternating electrodes with either a grid of holes, each just a few hundred nanometers across, or a single window of 6 × 10 µm, as depicted in figure 1. The total exposed area was the same for both types of electrodes, but whereas the windowed electrodes would allow free access to several microbes at a time, the nanoholes would preclude any direct contact between the electrode and the cell membrane.

Figure 1. Evidence for indirect, shuttle-mediated electron transfer. (a) The microbial fuel cell fashioned by Charles Lieber, Bradley Ringeisen, and colleagues featured two types of nanoelectrodes: One, shown at left, was exposed via nanoholes small enough to preclude contact with microbes; the other, shown at right, had large window exposures that allowed the microbes direct access. (b) Both types of electrodes generated nearly identical current, and that current vanished when shuttle molecules were flushed from the system, all of which suggests an indirect mechanism for electron transfer.
(Adapted from ref. 1.)
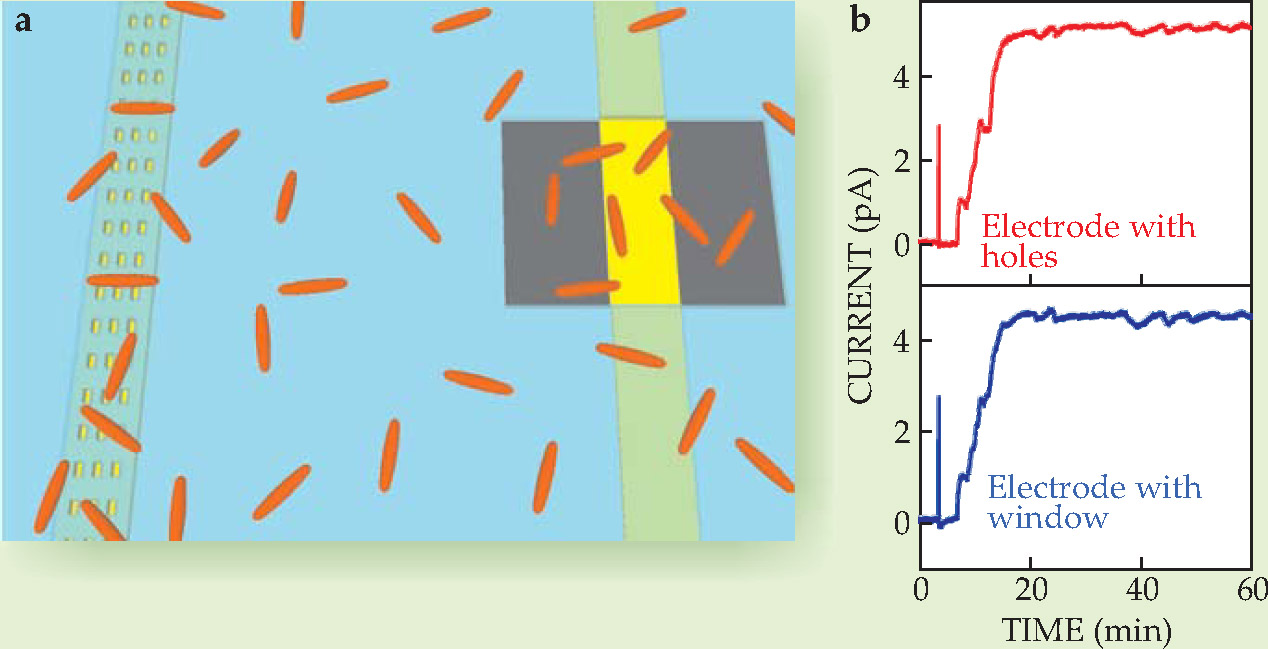
Upon introduction of the Shewanella culture, the two sets of electrodes responded almost identically: Within 20 minutes or so, both were producing a steady current of about 5 pA. “When you look at two electrodes that are within a diffusion length of molecules in solution,” says Lieber, “they show all the same fluctuations” — a strong indication that direct contact played little part in the microbe’s electron transfer.
Down to the wire?
But that seemingly neat picture fails to account for a key feature of Shewanella: Thanks to filament-like appendages that can stretch to several times the length of the cell itself, the microbe’s true reach extends well beyond its cell membrane. Gorby first encountered the appendages a few years ago while attempting to replicate the extreme growth conditions that microbes might encounter in underground and underwater environments. “When we limited the ability of organisms to get rid of electrons,” he explains, “their response was to make these appendages and hook themselves up into organized communities.”
Suspecting that the filaments might be involved in electron transfer, Gorby tested the idea with a scanning probe microscopy experiment and found that the appendages did indeed conduct across their diameter. But the more decisive test—demonstrating that the so-called bacterial nanowires could conduct along their length—proved more challenging than he had expected. El-Naggar’s expertise with nanoscale fabrication and measurements would provide a crucial piece to the puzzle.
Adapting techniques normally reserved for inorganic systems, Gorby and El-Naggar designed two experiments to test the lengthwise conductivity of Shewanella’s nanowires. 2 In the first, they used beam deposition to lay pairs of tiny platinum electrode contacts across the nanowires of microbes that had been chemically fixed with a preservative, dehydrated, and deposited on a layer of insulating silicon dioxide, as shown in figure 2(a). Sweeping the voltage across the electrodes and measuring the resulting current, they calculated a lengthwise resistivity of about 1 Ω·cm—on par with a moderately doped semiconductor. Using that value, the team estimates that just one nanowire would be more than sufficient to discharge the roughly 106 electrons produced per second by a Shewanella cell.

Figure 2. Evidence for direct, nanowire-mediated electron transfer. Mohamed El-Naggar, Yuri Gorby, and colleagues measured the conductivity of bacterial nanowires—filament-like microbial appendages—with two experiments. (a) In one, they fixed tiny pairs of platinum electrodes onto nanowires that lay across a bed of silicon dioxide, and they measured current as a function of an applied voltage drop. (b) In the other, they deposited microbes on a windowpane arrangement of SiO2 and gold surfaces, and then looked for nanowires that stretched across a Au-SiO2 interface. Applying the tip of a conducting-probe atomic force microscope to a point on the SiO2 side, such as the point indicated by the arrow in the inset, they passed current through the nanowire to the Au surface. Both methods returned a resistivity near 1 Ω·cm—on par with a moderately doped semiconductor and conductive enough to account for all of a bacterium’s electron transport.
((a) Image courtesy of Thomas Yuzvinsky and Greg Wanger.)
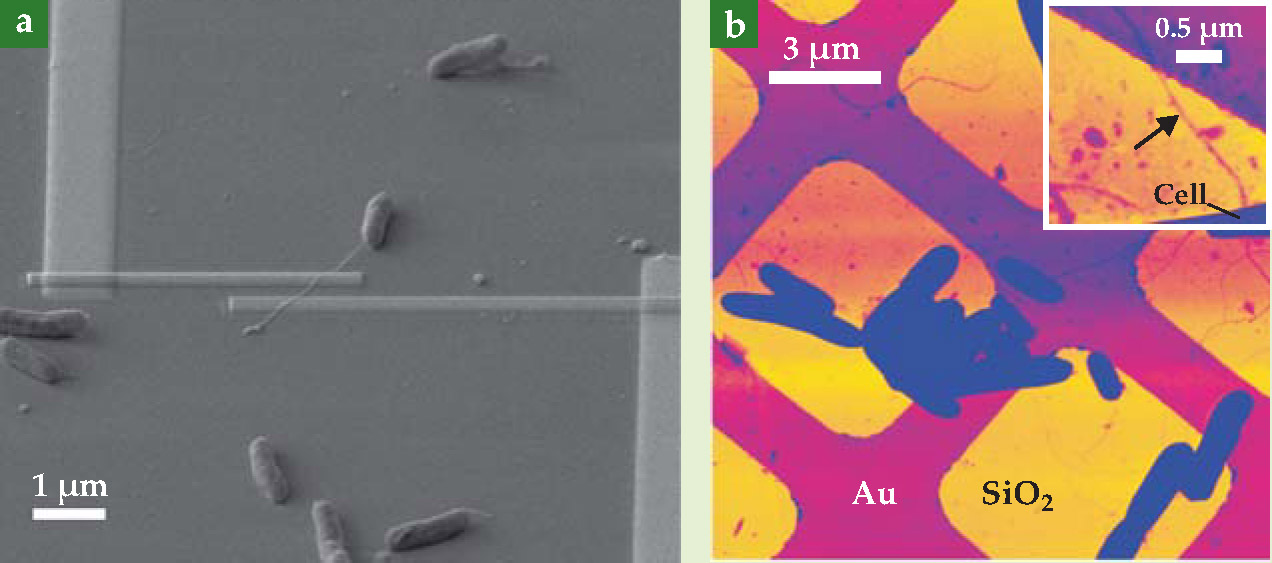
In theory, however, much of the measured resistance could have come from, say, the nanowire-platinum interfaces and not the nanowire itself; the two-electrode design affords no way to distinguish between the two. Thus, to crosscheck their result, the researchers deposited Shewanella microbes on a meshed grid of gold and silicon dioxide, as shown in figure
Wash, rinse, repeat
Up to tens of microns long and about 5-10 nm in diameter, the filamentous appendages are easily long enough and thin enough to have reached down into the nanoholes of Ringeisen and Lieber’s microbial fuel-cell experiment. Indeed, the researchers’ atomic force microscope images reveal several nanowires loitering near hole entrances. A follow-up experiment, however, confirmed that at least in their fuel cell, the shuttle molecules and not the nanowires were responsible for electron transfer.
The team arrived at that conclusion after flushing the flavin-rich extracellular, or supernatant, fluid from the fuel cell and replacing it with fresh medium. Explains Ringeisen, “We were expecting that if you flush the fuel cells to get rid of all the shuttling molecules, you’d still be able to get some current generation.” To the team’s surprise, however, the current vanished, even though phase-contrast images showed that nearly all the microbes at the windowed electrodes maintained direct contact. When the team reintroduced the supernatant fluid to the system, the current instantly returned to near its preflush levels.
Still, Ringeisen and Lieber are careful to note that their results can’t necessarily be generalized to all conditions. Both teams acknowledge that numerous factors—from the dissolved oxygen concentration to the type of electrode—are likely to affect a microbe’s respiratory behavior. The groups are now looking to collaborate to develop standardized, uniform protocols by which to grow and test their microbes.
Another challenge, assuming that the nanowires do conduct, is to identify the mechanism that enables them to do so. El-Naggar and Gorby suspect the appendages may comprise filaments of tightly packed metalloproteins called cytochromes. “Traditionally,” El-Naggar explains, “proteins are considered insulators or wide-bandgap semiconductors, but one can imagine that if there’s a unique organization of these electron-transport proteins coming together in a long chain, there may be a pathway for hopping of electrons.”
Ultimately, a better grasp of how microbes transfer electrons could help scientists identify ways to extract stronger currents from them. Existing microbial fuel cells are efficient but not powerful—they can’t yet compete with chemical, solid-oxide, or methanol fuel cells. Although we probably won’t be driving bacteria-powered cars in the near future, researchers may find more immediate use for the microbes in soil decontamination or remote underground sensing.
In any case, physicists and biologists alike can marvel at how nature has, yet again, achieved an engineering feat that humans aren’t likely to soon reproduce. “You can’t go in your nanofabrication facility and make a little 1-micron capacitor or a 1-micron battery that continuously produces electricity,” says Lieber. “But bacteria have figured out how to do this.”
References
1. X. Jiang et al., Proc. Natl. Acad. Sci. USA 107, 16806 (2010).
2. M. Y. El-Naggar et al., Proc. Natl. Acad. Sci. USA 107, 18127 (2010). https://doi.org/10.1073/pnas.1004880107




