Two-dimensional materials let protons pass
DOI: 10.1063/PT.3.2672
In a sense, graphene is the ultimate balloon. In a classic 2008 experiment, Paul McEuen and colleagues demonstrated that monolayers of the material are completely impermeable to air, argon, and helium. 1 They etched microchambers into the surface of an oxidized silicon wafer, filled the chambers with gas, and sealed them with graphene. Then they created a pressure difference so the graphene membranes bulged outwards. Over time, the bulges deflated—but the researchers established that all the escaping gas was seeping into the chambers’ glass walls or through the graphene–substrate seal. Essentially none of it crossed the graphene barrier itself.
Since then, theory and experiment alike have suggested that pristine graphene should block the passage of all atoms and molecules, including the smallest of atoms, hydrogen. Size isn’t the only determining factor: Leonidas Tsetseris and Sokrates Pantelides calculate that boron atoms should pass through graphene more readily than other, smaller atoms—but still not in any appreciable amounts under ambient conditions. 2
On the other hand, electrons can tunnel through graphene and other atomically thick layers with ease. Now Andre Geim (University of Manchester, UK), his students Sheng Hu and Marcelo Lozada-Hidalgo, and their collaborators have shown that protons, too, can pass through graphene monolayers. 3 For comparison, they tested two other two-dimensional materials: Molybdenum disulfide was impermeable to protons, but boron nitride let them through even more easily than graphene. The results position graphene and BN as promising new membrane materials for hydrogen fuel cells and hydrogen purification.
Permeable pores
The calculations that predict graphene’s impermeability to neutral H atoms should apply equally well to protons. But as Geim advised his students, “You shouldn’t think too much before trying new experiments; you can miss new phenomena otherwise.” So although theory predicted that protons wouldn’t pass through graphene, the Manchester researchers decided to see for themselves.
Gas-phase protons and H atoms are unstable at room temperature, but protons can be stabilized by electrolytes. So it was necessary to look for graphene’s proton permeability in the context of an electrochemical cell. The researchers experimented with many electrolytes, electrode materials, and setups. “With the benefit of hindsight,” says Lozada-Hidalgo, “we now know that all those systems were showing us the same results.” But at the time, their observations were complicated by numerous liquid-phase chemical reactions that proved impossible to control.
They finally settled on Nafion, a solid polymeric electrolyte that conducts protons but not electrons, and palladium hydride, an electrode material that converts electron current to proton current. Despite its name, palladium hydride isn’t an ionic compound of Pd cations and H anions; rather, it’s a metallic alloy of neutral Pd and neutral H that soaks up and releases H atoms like a sponge. Because it has no fixed composition, its formula is written PdHx. In an electrochemical cell, one PdHx electrode gives up some of its H to release protons into the electrolyte and electrons into the external circuit; the other electrode absorbs both protons and electrons to increase its H content.
The device setup is shown in figure 1. The researchers placed a piece of graphene or other 2D crystalline material over a tiny hole they’d etched in a layer of silicon nitride. Then they deposited Nafion and PdHx on both sides and applied an electric potential across the electrodes to see if the device would conduct electricity.

Figure 1. The electrical conductivity of this device depends on how readily protons can pass through the two-dimensional crystals (green) suspended over the silicon nitride membrane. Palladium hydride electrodes (black) convert the electron currents in the external circuit to proton currents in the Nafion electrolyte (light blue). (Adapted from ref.
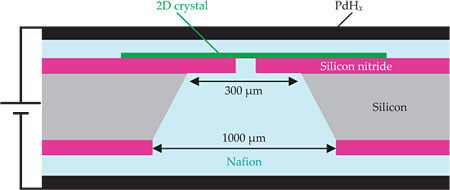
They wanted to make sure that any current they observed was the result of protons passing through the 2D material itself, not slipping through cracks or defects. So they examined their 2D crystals using scanning electron microscopy for large cracks and Raman spectroscopy for atomic-scale defects. To strengthen their case, they made several devices for each 2D material and found that the conductivity per unit area of the hole varied little.
Devices made with monolayer graphene conducted electricity moderately well, those made with monolayer BN even better, and those made with MoS2 not at all. That finding can be qualitatively explained by the materials’ electron-density distributions: Graphene and BN both have large pores with low electron density through which protons might pass with relative ease, but MoS2 doesn’t. In stacked layers of BN, those pores are aligned, whereas in multilayer graphene, they’re not. So it was little surprise that bilayer and trilayer BN devices both conduct, but bilayer graphene devices do not.
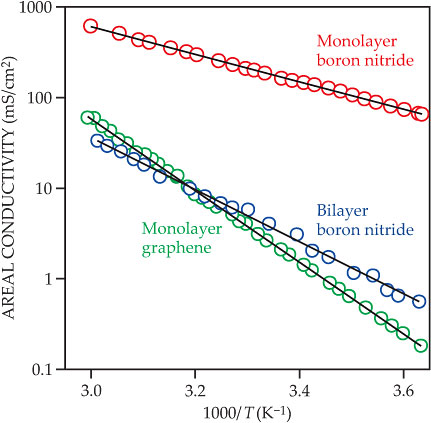
To better understand the conduction mechanism, the researchers varied the temperature T over a range of 0–60 °C. For all devices, conductivity was an exponential function of 1/T, as expected for a system of particles surmounting a fixed energy barrier. From the slopes of the lines in figure 2, it follows that the energy barrier is 0.78 eV for graphene, 0.30 eV for monolayer BN, and 0.61 eV for bilayer BN. The measured graphene barrier is considerably lower than theoretical predictions, which range from 1.2 eV to 2.2 eV. Because the barrier height depends on the specific way in which chemical bonds form and break as protons interact with the graphene electron cloud, predicting it accurately is no easy feat. The details of the true transport pathway remain unknown.

Figure 2. Temperature dependence of the conductivity of three proton-permeable materials. The conductivity per unit area is an exponential function of the reciprocal of the temperature T. (Adapted from ref.
Moving hydrogen
Membranes that are selectively permeable to protons could be a boon to hydrogen-based technologies. In hydrogen fuel cells, for example, the reactants, O2 and H2, need to be kept separate so they don’t explode. The reactant molecules are catalytically dissociated on opposite sides of a membrane, then the H+ ions pass through the membrane to react with O atoms and produce water. (See the article by Michael Eikerling, Alexei Kornyshev, and Anthony Kucernak, Physics Today, October 2006, page 38
The current favorite material for fuel-cell membranes is Nafion, but its high cost makes it more suitable for use in satellites and other high-budget applications than in mass-market products. Furthermore, it can’t operate at temperatures higher than 80 °C, and it requires constant humidification, even when the fuel cell is turned off. Of the alternative materials that have been studied, most are polymers similar to Nafion. 4 Graphene or BN could overcome Nafion’s difficulties. Graphene, at least, is known to be stable under fuel-cell conditions up to 400 °C, and from an extrapolation of the data in figure 2, it should have more than the necessary conductivity at just 110 °C.
Another potential application is to hydrogen purification. That’s currently done using membranes of a Pd alloy, which are expensive and prone to cracks. The Manchester researchers have already demonstrated that graphene can purify hydrogen on a small scale: They built a device similar to the one in figure 1, but with the Nafion and PdHx on only one side, and they allowed protons passing through the graphene to escape into a vacuum and combine to form H2.
Scaling up either application would require overcoming a host of technical challenges. “But we believe that the incentive is there,” says Hu. “Hopefully, experts around the world will take interest.”
References
1. J. S. Bunch et al., Nano Lett. 8, 2458 (2008). https://doi.org/10.1021/nl801457b
2. L. Tsetseris, S. T. Pantelides, Carbon 67, 58 (2014). https://doi.org/10.1016/j.carbon.2013.09.055
3. S. Hu et al., Nature 516, 227 (2014). https://doi.org/10.1038/nature14015
4. R. Devanathan, Energy Environ. Sci. 1, 101 (2008).https://doi.org/10.1039/b808149m
More about the authors
Johanna L. Miller, jmiller@aip.org




