Tailor-made molecules grow into identical carbon nanotubes
DOI: 10.1063/PT.3.2536
Carbon nanotubes (CNTs)—graphene sheets wrapped into hollow cylinders a nanometer or two in diameter—have inspired a vast array of proposed applications based on their many extraordinary characteristics. They’re mechanically strong, flexible, and lightweight. And their structure-dependent electronic and optical properties make them ideal components for FETs, photonic systems, and more. (See the article by Phaedon Avouris, Physics Today, January 2009, page 34
A CNT’s atomic structure is defined by a pair of indices (n,m), which together determine its diameter and the orientation of the graphene lattice with respect to the tube’s axis. (See the article by Cees Dekker, Physics Today, May 1999, page 22
The CNT community has grappled for decades with the challenge of producing nanotubes on demand with a particular predefined n and m. Standard methods of synthesis always produce a mix of many structures. Some research groups have succeeded in reducing the number of structures in that mix. 1 Others have focused on postsynthesis sorting of nanotubes based on their physical or chemical properties. 2
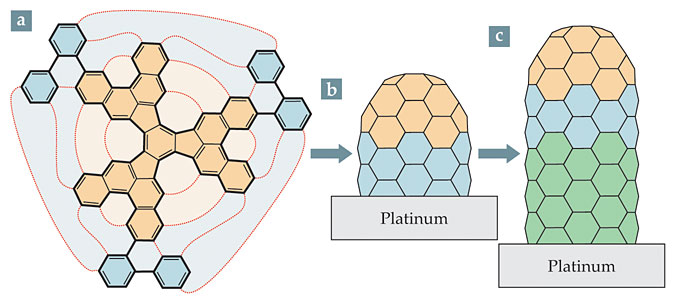
Surface scientist Roman Fasel (Swiss Federal Laboratories for Materials Science and Technology), organic chemist Konstantin Amsharov (now at the University of Erlangen-Nuremberg in Germany), and their colleagues 3 have now taken a big step forward by synthesizing, for the first time, a small quantity of CNTs all with indices (6,6). To start, the researchers implemented a difficult, 10-step chemical synthesis to create the precursor molecule shown in figure 1a. They deposited the precursors on a platinum surface and induced them to fold into cap-shaped seeds like the one shown in figure 1b. Then, using ethylene or ethanol gas as a source of new carbon atoms, they grew the seeds into longer CNTs, as shown in figure 1c, that always conformed to the structure of the seeds.

Figure 1. Nanotubes by design. The C96H54 precursor (a), placed on a flat platinum surface, undergoes cyclodehydrogenation to break the carbon–hydrogen bonds around its edges and form new carbon–carbon bonds, as indicated by the red lines. As a result, a cap-shaped seed (b) is created. Introducing carbon-containing gases, such as ethanol or ethylene, results in the addition of C atoms at the C–Pt interface (c), and the carbon nanotube begins to grow. (Adapted from ref.
Pop-up precursors
The conventional synthesis of CNTs proceeds via a root-growth mechanism: Carbon atoms adhere to the surface of a catalytic metal nanoparticle and nucleate into an endcap structure, which then grows into a tube. 4 Considering that mechanism, Amsharov speculated that by replacing the randomly formed endcaps with precisely synthesized ones, one could exert an equally precise control over the CNTs’ structure. There were three hurdles to overcome: designing and producing suitable endcap molecules, getting those molecules to grow into CNTs, and preventing randomly nucleated endcaps from forming at the same time.
Wet chemical synthesis of three- dimensional cap-shaped molecules—so-called buckybowls—is extremely difficult. A few such hemispherical structures have been made in the lab, but not with the precision and flexibility that would be required for controllably seeding CNT growth. On the other hand, the toolkit of techniques for making flat polycyclic aromatic hydrocarbons—aggregations of benzene rings—is well developed. On a Pt surface, flat precursors can be made to undergo cyclodehydrogenation to break the carbon–hydrogen bonds around the molecule’s periphery, replace them with new carbon–carbon bonds, and create a 3D structure. Amsharov and colleagues developed suitable-looking precursors for several different CNT structures; 5 their first attempt at a (6,6) precursor was the C60H30 structure shown in tan in figure 1a.
The researchers deposited the molecules on a metal surface and dehydrogenated them. But when they tried to grow the seeds into longer CNTs, the molecules fell apart. So they tried again: Knowing that (6,6) nanotubes themselves are stable up to high temperatures, they extended their precursor by adding the benzene rings shown in blue, which would yield an endcap with a short segment of nanotube already attached.
The expanded precursor, C96H54, is floppy, and sometimes it falls on the Pt surface with one or more of the blue arms in the wrong position. Fortunately, the right position is also the energetically favored one, and each arm adopts it far more than half the time. In fact, scanning tunneling microscopy (STM) of the surface-deposited precursors showed that at least half the molecules had all three of their arms in the right position. More importantly, dehydrogenating the molecules that had one or more of their arms out of place produced structures that couldn’t possibly grow into nanotubes, so they were no threat to the purity of the (6,6) sample.
In conventional nanotube synthesis, metal nanoparticles are needed to catalyze the nucleation of the endcaps. In the new approach, nanoparticles are no longer necessary, and in fact, the cyclodehydrogenation step proceeds much more smoothly on a flat Pt surface. Fasel and Amsharov worried that growing CNTs on a flat surface would require a much higher temperature than the usual 600–800 °C, if it could be done at all. But to their surprise, CNTs grew at 500 °C. That lower temperature, the researchers guess, helped to suppress the nucleation of new endcaps with uncontrolled structure. It also kept structure-altering defects from forming in the nanotubes as they grew.
Testing uniformity
To check the structure of the CNTs, Fasel and colleagues started with high-resolution STM. Every nanotube they imaged, like the one in figure 2a, had a (6,6) structure. But it’s not possible to characterize the whole sample by imaging nanotubes one by one—especially because STM images can reveal the structures only of CNTs oriented parallel to the surface. Because the nanotubes grew perpendicular to the surface, the STM images showed tubes that had toppled over.

Figure 2. Characterizing nanotubes. High-resolution scanning tunneling microscopy (a) reveals the (6,6) structure of individual nanotubes, as shown by the overlaid ball-and-stick structure. (b) Raman spectroscopy probes the structure of all the nanotubes in an area of several square microns. The radial-breathing-mode peak is as narrow as those observed for isolated single nanotubes. The higher-frequency G band arises from vibrational modes in which adjacent atoms move in opposite directions. (Adapted from ref.
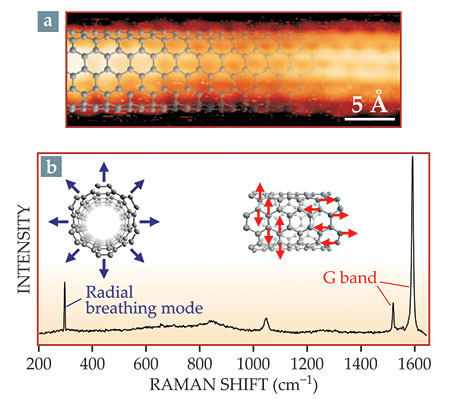
For a larger-scale characterization, then, the researchers turned to Raman spectroscopy to classify nanotubes based on their vibrational modes. Prominent CNT modes include the low-frequency radial breathing mode, shown by the blue arrows in figure 2b, and the high-frequency G band. Because the G band comprises two distinct vibrational motions, both shown by the red arrows, it’s split into two peaks. The exact frequency of each mode depends on a nanotube’s structure, so for a typical CNT sample comprising a variety of structures, Raman peaks are broad. But Fasel and colleagues saw sharp peaks. Their radial-breathing-mode peak, in particular, is the same width as is seen in the Raman spectrum of single, isolated nanotubes. Moreover, when the Raman laser beam was widened to sample a larger area, the peak widths didn’t change. The researchers concluded that all of their CNTs had the same structure—which, based on the STM images, would have to be (6,6).
Some experiments, however, have found that (n,n) CNTs, because of their symmetry, have only a single G-band peak, 6 whereas the spectrum in figure 2b clearly shows two. Fasel speculates that the second peak might arise either from the endcaps themselves or from especially short (6,6) CNTs that lack the symmetry of their longer counterparts. “It’s an open question,” he says. “We will definitely look into it in the near future.”
Scaling up
For their proof-of-principle demonstration, the researchers didn’t attempt to measure the total mass or number of CNTs they’d made; neither STM nor Raman spectroscopy is well suited to that purpose. But the researchers anticipate that the process can be scaled up to bulk proportions. Although the precursor synthesis is complicated and expensive, a single precursor molecule can grow a CNT many microns long—so a kilogram of (6,6) nanotubes might eventually be grown with just milligrams of the precursor.
A tougher challenge may be making more efficient use of the Pt surface area. As is, CNTs grow sparsely on the Pt surface; even if the precursor molecules are deposited more densely, relatively few grow into long nanotubes. Understanding the processes responsible for the sparse growth could offer routes to increase the yield.
The (6,6) structure was a natural choice for a first demonstration because of its high symmetry. Precursor molecules are built out of hexagonal benzene rings, and it’s relatively easy to make precursors with threefold or sixfold symmetry. But the techniques of organic chemistry allow for other structures—for example, by making the three arms of the precursor different shapes. The researchers are currently working to extend their method to other (n,m) pairs. “This may be challenging,” says Fasel, “but it will ultimately be limited only by the imagination and skills of the synthetic chemist.”
References
1. See, for example, M. He et al., Sci. Rep. 3, 1460 (2013); https://doi.org/10.1038/srep01460
F. Yang et al., Nature 510, 522 (2014). https://doi.org/10.1038/nature134342. M. C. Hersam, Nat. Nanotechnol. 3, 387 (2008). https://doi.org/10.1038/nnano.2008.135
3. J. R. Sanchez-Valencia et al., Nature 512, 61 (2014). https://doi.org/10.1038/nature13607
4. J. Gavillet et al., Phys. Rev. Lett. 87, 275504 (2001). https://doi.org/10.1103/PhysRevLett.87.275504
5. A. Mueller, K. Y. Amsharov, Eur. J. Org. Chem. 2012, 6155 (2012). https://doi.org/10.1002/ejoc.201200841
6. E. H. Hároz et al., Phys. Rev. B 84, 121403 (2011).https://doi.org/10.1103/PhysRevB.84.121403
More about the authors
Johanna L. Miller, jmiller@aip.org




