Semiconductor crystals achieve record thermal conductivity
DOI: 10.1063/PT.3.4014
When a laptop or cell phone heats up from overuse, it’s not just uncomfortable for the user. That excess heat also damages the circuitry and reduces the device’s performance, energy efficiency, and life span. As modern electronic components are made smaller, their resistance rises. Heat dissipation has become a critical technological challenge for next-generation items, including microprocessors and integrated circuits, LEDs, and high-powered RF products.
Materials with high thermal conductivity help dissipate heat and improve the performance and reliability of those devices. However, developing a passive cooling option that is both cost-effective and reliable has been difficult. With a thermal conductivity of 2000 W/m·K, diamond is the best-known material for cooling. But it is expensive, has slow synthesis rates, and is of varying quality. Integrating diamond with silicon and other industrial semiconductors is also challenging because those materials have different thermal expansion coefficients.
Among metals, copper has the highest thermal conductivity at room temperature, 400 W/m·K, and is commonly used in industry for dissipating heat in electronics. Among binary compounds, silicon carbide, at 350 W/m·K, is favored for thermal management in personal electronics. To control the high-density heat generated in small, power-hungry devices, however, the ideal material would have a thermal conductivity at least as high as diamond’s.
Now three research groups have synthesized boron arsenide (BAs) crystals that have a thermal conductivity of more than 1000 W/m·K at room temperature, exceeding that of all other materials except diamond.
1–3
BAs still has a long way to go in terms of size, consistency, and cost before it could be used in an actual device. Nonetheless, the new research vindicates theoretical predictions that have not been verified until now and might lead to the discovery of new practical materials. Figure
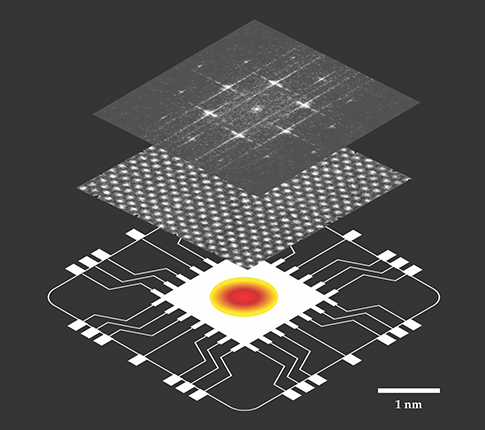
Figure 1.

Concept of thermal management and a boron arsenide crystal. This illustration shows a computer chip with a hot spot at the center (bottom). A high thermal conductivity material like BAs could help dissipate the chip’s heat. A transmission electron microscope image shows the atomically resolved lattices of a BAs crystal grown by UCLA researchers (middle). The electron diffraction pattern of the BAs crystal gives rise to clear bright spots, whose symmetry indicates the periodic structure and lack of defects in the crystal (top). (Image courtesy of Yongjie Hu.)
Good vibrations, in theory
In copper and other metals, nearly all heat conduction occurs via charge carriers. In diamond and similar nonmetals, which have no mobile electrons, heat is carried by phonons, or vibrations in the crystal lattice. When phonons scatter off each other, they slow down. A crystal structure with low phonon scattering has little thermal resistance and thus high conductivity.
In the early 1970s, Glen Slack of General Electric established with experiments and theory that high thermal conductivity in a nonmetallic solid required a simple crystal made of tightly bonded, lightweight elements, such as carbon. 4
Theoretical predictions in 2013 by David Broido at Boston College and Lucas Lindsay and Thomas Reinecke at the US Naval Research Laboratory suggested that the conventional criteria for high thermal conductivity, as described by Slack, were incomplete.
5
High thermal conductivity could be possible not only in crystals comprised of lightweight elements but also in crystals with one heavy and one light atom. (See the article by Ilari Maasilta and Austin Minnich, Physics Today, August 2014, page 27
In particular, the team’s theory predicted that thermal conductivity in BAs could come close to that of diamond. The mass difference between boron and arsenic creates an energy gap between the two types of phonons, optical and acoustic, in the crystal. Optical phonons arise from out-of-phase vibrations between neighboring atoms, while acoustic phonons arise from in-phase vibrations. That large energy gap makes it harder for phonons to interact with each other. They can therefore travel efficiently through the crystals, resulting in greater thermal conductivity.
In 2017 Lindsay and Xiulin Ruan and his colleagues at Purdue University revised the 2013 theoretical results. The new work predicted that thermal conductivity of BAs should actually be close to 1300 W/m·K in defect-free crystals. 6 It accounted for scattering among combinations of up to four phonons, while the earlier work only considered combinations of three phonons. Although three-phonon combinations dominate scattering in crystals that have a small energy gap, they cannot occur across the large energy gap in BAs. Including a fourth phonon increases the number of possible combinations and is important to consider for determining the scattering in BAs.
Almost defect-free
Synthesizing high-quality BAs crystals in bulk is difficult. Arsenic’s high volatility tends to introduce defects and possible formation of unwanted atom combinations. Those defects and impurities dramatically reduce thermal conductivity by narrowing the energy gap between the phonons.
In 2015 Zhifeng Ren at the University of Houston and Bing Lv, then an assistant professor at the University of Houston, and colleagues synthesized BAs crystals using chemical vapor transport, a technique in which solids are vaporized and deposited as crystals. Unfortunately, despite the technique’s promise, the crytals were riddled with defects. Yongjie Hu, then a postdoc at MIT, measured a thermal conductivity of only 190 W/m·K.
Now three teams led respectively by Ren, Lv, and Hu have each succeeded in optimizing the technique to synthesize crystals with thermal conductivity of at least 1000 W/m·K at room temperature.
The teams vaporized solid boron and arsenic in chambers that contained hot and cold temperature regions. Different nonreacting gaseous agents carried the two elements from the hot end to the cold, where the two combined to form crystals. Using a technique called time-domain thermoreflectance, each team measured the thermal conductivity on micrometer-scale regions of the surface.
Lv, now at the University of Texas at Dallas, and David Cahill of the University of Illinois at Urbana-Champaign measured room-temperature thermal conductivity of 0.5 mm crystals. 1 Repeated measurements on 50 samples, grown in different batches, revealed variations in thermal conductivity. The researchers used Raman spectroscopy, x-ray diffraction, and transmission electron microscopy to study the BAs samples for defects. They found a correlation between the thermal conductivity and the defect concentration, with the lowest-defect samples achieving the highest conductivity (1000 W/m·K).
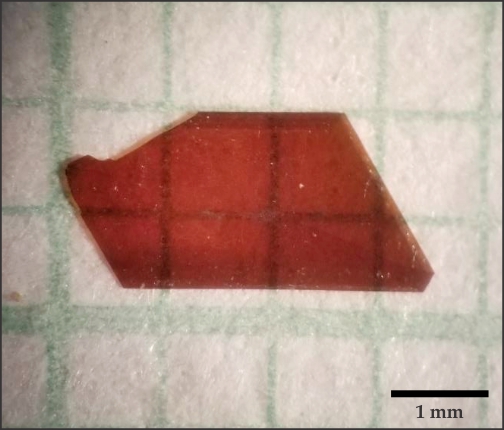
Ren’s team at the University of Houston grew larger crystals up to 4 mm in size (see figure
Figure 2.

A 4 mm single boron arsenide crystal grown by researchers at the University of Houston. (Image courtesy of Zhifeng Ren.)
Hu’s team at UCLA developed very high-quality 2 mm crystals that had undetectable defects. They also recorded the highest thermal conductivity of the three groups, 1300 W/m·K, as predicted by theory. 3 Local measurements were consistent across the entire large crystal. Hu’s group took several years to learn how to control the synthesis precisely enough to make samples approaching the defect-free limit. “Our study exemplifies the power of combining experiments and ab initio theory in new materials discovery,” says Hu.
Lab to market
For BAs to be useful in electronic devices, the researchers still must find ways to make larger samples and more of them. A semiconductor wafer inside a mobile phone, for example, needs to be several centimeters in scale. Scientists need to understand and control the types of defects present in the materials and to make the synthesis process more robust.
Besides high thermal conductivity, BAs has other desirable properties for thermal control, including chemical inertness and a coefficient of thermal expansion similar to that of silicon. Still, Cahill warns that “we have no idea if boron arsenide can be produced at a practical volume. Maybe eventually, but would it be cost-effective? We just don’t know.”
Shi adds, “It remains a grand challenge to understand the defect-formation mechanism.” Now that scientists have a method for synthesizing nearly defect-free crystal, they can also introduce specific defects. Boundary and point defects could affect phonon transport and thermal conductivity in different ways. In that way, BAs offers the opportunity to study the origin of high thermal conductivity itself.
References
1. S. Li et al., Science 361, 579 (2018). https://doi.org/10.1126/science.aat8982
2. F. Tian et al., Science 361, 582 (2018). https://doi.org/10.1126/science.aat7932
3. J. S. Kang et al., Science 361, 575 (2018). https://doi.org/10.1126/science.aat5522
4. G. A. Slack, J. Phys. Chem. Solids 34, 321 (1973). https://doi.org/10.1016/0022-3697(73)90092-9
5. L. Lindsay, D. A. Broido, T. L. Reinecke, Phys. Rev. Lett. 111, 025901 (2013). https://doi.org/10.1103/PhysRevLett.111.025901
6. T. Feng, L. Lindsay, X. Ruan, Phys. Rev. B 96, 161201 (2017). https://doi.org/10.1103/PhysRevB.96.161201




