Optical absorption of single molecules observed by three groups
DOI: 10.1063/1.3528997
Chemistry is, for the most part, a field of ensemble measurements. Large numbers of molecules acting together produce effects that are macroscopically observable. But in many cases, such as the study of inhomogeneous systems or of processes with complex time dependence, observing molecules one by one can provide information that an ensemble measurement cannot.
Much of the flavor of single-molecule optical detection—a less invasive technique than, for example, scanning tunneling microscopy—dates back to 1976, when Tomas Hirschfeld used an optical microscope to observe proteins tagged with tens of fluorescent molecules. 1 True single-molecule detection came in 1989-90, achieved independently by William Moerner and Michel Orrit and their colleagues. 2 Both worked at cryogenic temperatures, at which molecular spectral peaks are much stronger and sharper. Room-temperature detection came shortly thereafter and has since facilitated a multitude of advances in biology, materials science, and other fields. Among the more striking recent developments is the real-time sequencing of a strand of polymerizing DNA. 3
So far, room-temperature detection of single molecules has relied on fluorescence, the light emitted by an optically excited molecule as it relaxes back to its ground state. Now, three groups working independently have detected single molecules by their optical absorption. Since many molecules fluoresce only weakly or not at all but all molecules absorb, the new work paves the way for single-molecule study of a much wider class of substances.
Each group used a different method. Vahid Sandoghdar (ETH Zürich, Switzerland) and colleagues detected the absorption directly. 4 Orrit (Leiden University, the Netherlands) and colleagues scattered light off the hot spot created around a molecule that rapidly converts light to heat. 5 And Sunney Xie (Harvard University) and colleagues used a nonlinear technique to extract the absorption signal from a high-frequency amplitude oscillation of a probe laser beam. 6
Direct detection
To directly detect a weak absorption, one must measure the tiny changes in a light beam’s intensity as it’s attenuated by the sample. (In contrast, a weak fluorescence produces a low-intensity signal with almost no background.) A typical molecular absorption cross section is on the order of 0.1 nm2, which means that a tightly focused laser beam 300 nm in diameter is attenuated by about one part per million.
Sandoghdar and colleagues became interested in pushing the limit of direct fluorescence-free measurements in 2003. They had been working with small nanoparticles, which gave absorption signals of about one part in 104. Their calculations suggested that their method should also be sensitive to single molecules, so they decided to give it a try. As Sandoghdar notes, “The main challenge was to believe that it’s doable.”
The experimental setup is shown schematically in figure 1. The laser beam was split into two parts: a probe beam, which passed through the sample, and a reference beam, which didn’t. Detecting the two beams simultaneously and comparing their intensities helped to isolate the absorption signal from the laser’s intensity fluctuations.

Figure 1. Direct detection, used by Vahid Sandoghdar and colleagues.
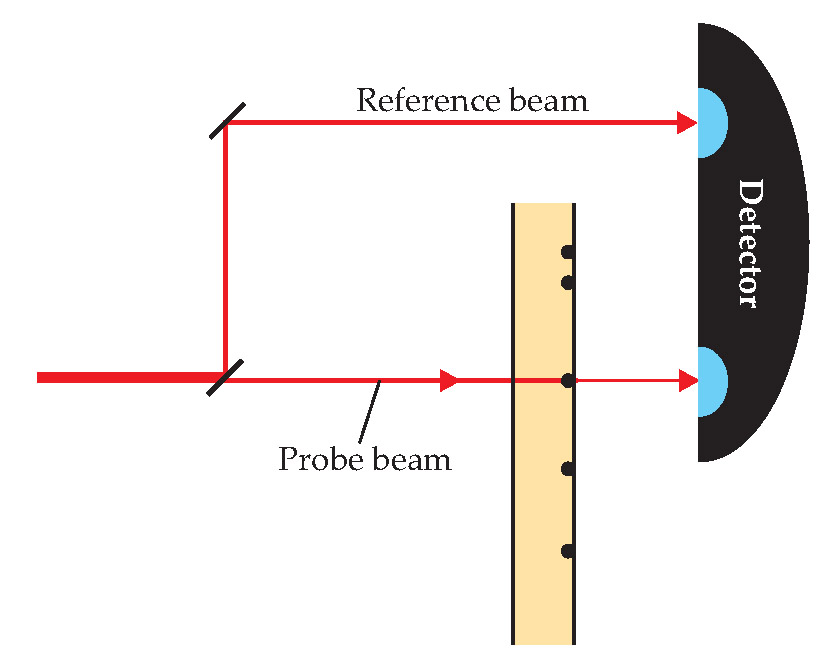
The sample consisted of a low concentration of dye molecules dispersed in a polymer layer and affixed to a glass slide; a piezoelectric stage drew the slide back and forth through the probe beam. For this proof-of-principle experiment, the researchers used only fluorescent molecules, so they could monitor the fluorescence and absorption signals simultaneously. After several seconds of illumination, a molecule’s fluorescence signal would abruptly disappear: The molecule had “photobleached,” or undergone a photochemical reaction that changed its light-absorbing properties. The absorption signal always disappeared at the same time, evidence that the two signals were coming from the same molecule.
Actually, Sandoghdar and colleagues measured not absorption but extinction: Their method doesn’t distinguish between absorption and scattering. That could be a disadvantage, since surface roughness at a material interface could scatter enough light to overwhelm the molecular signal. The researchers overcame that difficulty by choosing materials with matched refractive indices. Sandoghdar believes that sensitivity to scattering is also an advantage, since it enables the study of entities such as proteins that scatter more light than they absorb.
Photothermal imaging
Orrit and colleagues used a two-laser technique, sketched in figure 2, based on the photothermal effect. The first, or heating, laser is tuned to a molecular absorption, and the second, or probe, operates at a frequency at which the sample is transparent. When light from the heating laser is absorbed by a molecule, its energy is converted to heat, which changes the refractive index of the surrounding material. Light from the probe laser then scatters off the refractive-index inhomogeneity, a “thermal lens,” and is collected by a detector.

Figure 2. Photothermal imaging, used by Michel Orrit and colleagues.
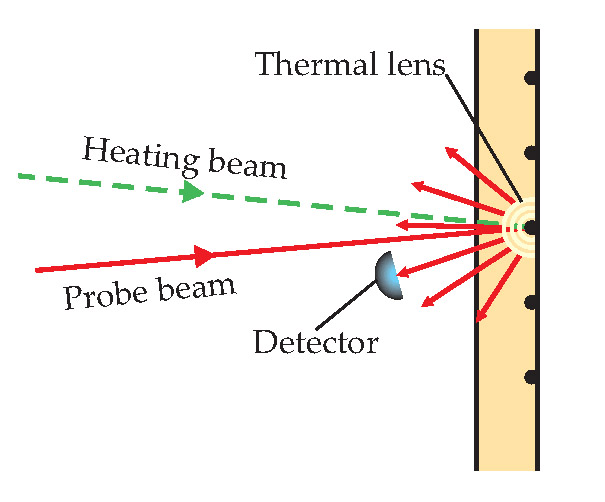
For photothermal imaging to work, the heat needs to be imparted to a material whose refractive index changes significantly with temperature. Water at room temperature doesn’t fit the bill: 20 °C is too close to 4 °C, the temperature at which water’s density is maximized— and therefore flat—as a function of temperature. So Orrit and colleagues chose glycerol as a solvent instead.
Like Sandoghdar’s group, Orrit and colleagues turned to nanoparticles to begin exploring the limits of their technique. Since the photothermal signal depends on the amount of heat absorbed and not just the strength of the absorption, they could use nanoparticles to simulate the more weakly absorbing molecules simply by turning down the heating laser’s power. They then tried to detect some weakly fluorescent molecules but found that those all photobleached too quickly. A non-fluorescent molecule—a dye designed to quench fluorescence in biochemical experiments—gave better results and, surprisingly, seemed not to photo-bleach at all. Without fluorescence to guide them, the researchers took a different approach to verifying that their signal was due to molecular absorption: They recorded photothermal images of several samples with different amounts of dye and showed that the number of bright spots in the image was proportional to the dye concentration.
Ground-state depletion
Xie’s group was among the first, in the 1990s, to detect and image single molecules at room temperature. (See Physics Today, May 1994, page 17

Figure 3. Ground-state depletion, used by Sunney Xie and colleagues.
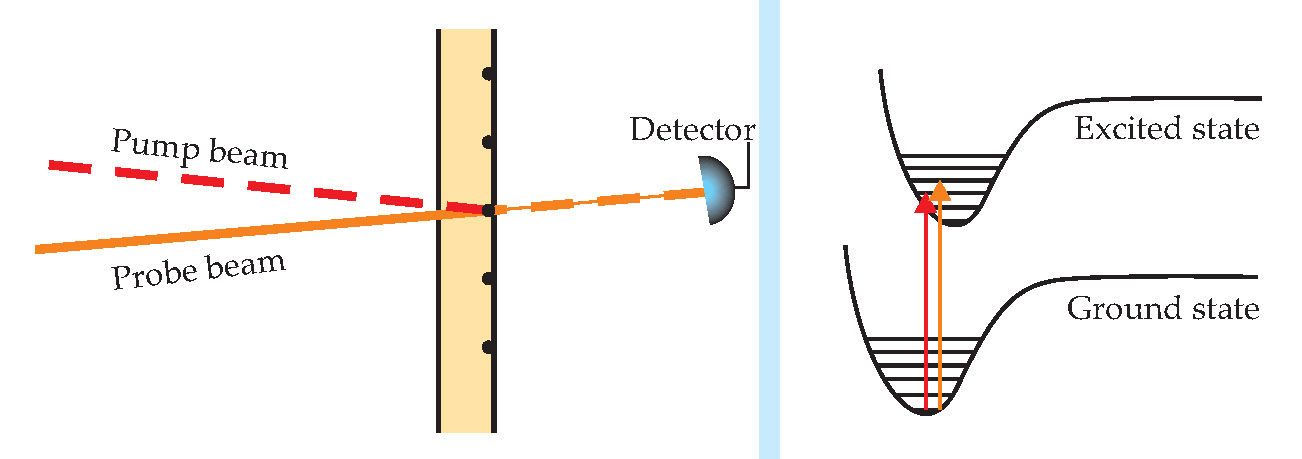
Using a lock-in amplifier, the researchers isolated the probe beam’s high-frequency fluctuations. Since the amplitude noise of a continuous-wave laser beam scales like the reciprocal of the fluctuation frequency, it has almost no effect on the observed high-frequency signal. Likewise, scattering due to surface roughness has no effect on the high-frequency fluctuations, so there is no need to choose materials with matched refractive indices.
Like the Sandoghdar group, Xie and colleagues have looked only at fluorescent molecules so far. They, too, found that the absorption and fluorescence signals appeared together, and when the fluorescence disappeared due to photobleaching, so did the absorption.
What’s next?
To gather enough data to detect a molecule, all three techniques require relatively long averaging times, on the order of 1 s. Since free-floating molecules don’t stay in place for that long, all three groups studied molecules immobilized on surfaces. Reducing the averaging time will be key to making the single-molecule absorption techniques suitable for many applications.
But all three measurements are nearing the shot-noise limit, where the main source of error is the statistical fluctuation of the number of photons in the laser beam, so strategies to improve the sensitivity are far from obvious. Xie supposes that progress might come through the use of nonclassical light states, such as squeezed light, for which certainty in the phase is sacrificed in favor of certainty in the amplitude, or entangled light, in which the photon counts of two beams are better correlated than if the beams had been produced independently. Orrit, for his part, advises patience: “For single-molecule fluorescence, which is a more straightforward technique, it took the best part of 20 years to generalize it to more complex conditions. I expect a similar delay for absorption.”
References
1. T. Hirschfeld, Appl. Opt. 15, 2965 (1976). https://doi.org/10.1364/AO.15.002965
2. W. E. Moerner, L. Kador, Phys. Rev. Lett. 62, 2535 (1989)https://doi.org/10.1103/PhysRevLett.62.2535
M. Orrit J. Bernard, Phys. Rev. Lett. 65, 2716 (1990).https://doi.org/10.1103/PhysRevLett.65.27163. J. Eid et al., Science 323, 133 (2009). https://doi.org/10.1126/science.1162986
4. P. Kukura, M. Celebrano, A. Renn, V. Sandoghdar, J. Phys. Chem. Lett. 1, 3323 (2010).
5. A. Gaiduk, M. Yorulmaz, P. V. Ruijgrok, M. Orrit, Science 330, 353 (2010). https://doi.org/10.1126/science.1195475
6. S. Chong, W. Min, X. S. Xie, J. Phys. Chem. Lett. 1, 3316 (2010).
More about the authors
Johanna L. Miller, jmiller@aip.org




