Multiple exciton generation enhances a working solar cell
DOI: 10.1063/PT.3.1426
As solar electricity continues its progress toward widespread commercial viability, researchers are on the lookout for ways to make photovoltaic cells cheaper, more efficient, or both. Increasing efficiency will eventually require exceeding the Shockley–Queisser limit—the maximum power-conversion efficiency of a semiconductor solar cell with a single bandgap, one electron–hole pair excited per photon, and no multiphoton processes.
In such a cell, photons with energies less than the bandgap have no effect, and their energy is lost. More energetic photons excite electrons from the valence band to the conduction band, but the charge-carrier pairs quickly relax to the energy of the bandgap, and any excess energy is lost as heat. (See the article by George Crabtree and Nathan Lewis in PHYSICS TODAY, March 2007, page 37
There are several ways to exceed the Shockley–Queisser limit by violating one of its premises. For example, a multijunction solar cell—a stack of two or more semiconductor junctions with different bandgaps—can capture more energy from the high-energy photons without losing the lower-energy ones. But multijunction cells are so expensive to make that they’re practical only for applications in which power per unit weight is more important than cost, such as on spacecraft.
Now, a different premise of the Shockley–Queisser limit has been violated. Arthur Nozik, Matthew Beard, and their colleagues at the National Renewable Energy Laboratory (NREL) in Golden, Colorado, have produced solar cells that can yield an external quantum efficiency (EQE) greater than 100%—that is, an output current of more than one charge-carrier pair per incident photon—for light wavelengths present in the solar spectrum. 1 (A high EQE doesn’t necessarily imply a high power-conversion efficiency, since power conversion depends on both output current and output voltage.) The key process, multiple exciton generation (MEG), had been predicted by Nozik in the early 2000s to occur in semiconductor nanocrystals (also called quantum dots). 2 And unlike multijunction cells, nanocrystal-based cells should be inexpensive to produce.
Size matters
Multiple exciton generation is a close cousin of impact ionization, a bulk-semiconductor phenomenon in which a fast-moving electron collides with another electron and excites it to the conduction band. Impact ionization doesn’t have much of an effect on bulk-semiconductor solar cells, because the highly excited electrons are far more likely to lose their energy by exciting phonons. Still, in 1998 an international team of researchers demonstrated an EQE of 128% for a bulk-silicon photodiode. 3 But they used photons of energy 7.7 eV (wavelength 162 nm)—seven times the Si bandgap and far outside the solar spectrum.
Nozik wondered whether the same process in nanocrystals might be more efficient. He had been inspired by work on fluorescence blinking in quantum dots (see the article by Fernando Stefani, Jacob Hoogenboom, and Eli Barkai in PHYSICS TODAY, February 2009, page 34

Figure 1. When a solar cell absorbs a photon with far greater energy than the bandgap, the photon creates an energetic electron–hole pair. In a bulk-semiconductor cell (left), the electron and hole quickly relax to the edges of the conduction and valence bands, losing much of their energy to heat. In a nanocrystal-based cell (right), on the other hand, the conduction-band electronic states are more widely spaced, so phonon-mediated cooling is slowed, and the electron has time to excite a second electron. (Adapted from ref.
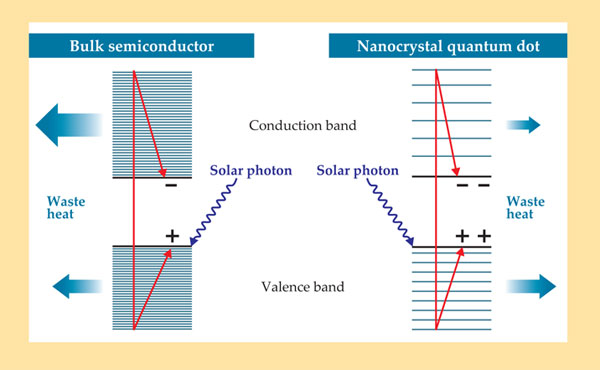
Nozik and his colleagues set out to look for MEG in III–V semiconductor nanocrystals. But the first successful observation came in 2004 from Victor Klimov’s group at Los Alamos National Laboratory. 4 Nozik had been looking in III–V semiconductor nanocrystals; Klimov was the first to look at lead compounds, which were a better choice. Researchers at NREL confirmed Klimov’s work and extended it to other materials. The early MEG experiments all used spectroscopic probes, and they were not without controversy. The spectroscopic results depend on several system properties that are difficult to analyze and control, so other groups had trouble reproducing the Los Alamos and NREL groups’ observations.
Collecting charges
Apart from the lingering question of whether MEG occurs at all, there remained the challenge of making use of it in a solar cell. Getting the charge carriers out of a nanocrystal-based solar cell is not as straightforward as getting them out of a bulk-semiconductor cell, because wires cannot be attached to each individual nanocrystal. In 2010 Bruce Parkinson and colleagues at the University of Wyoming created a photovoltaic device, 5 based on a monolayer of lead sulfide nanocrystals, with an internal quantum efficiency (IQE, the number of carrier pairs in the output current per absorbed photon) of more than 100%. Each nanocrystal in a monolayer offers a direct route to the external circuit. But a monolayer doesn’t absorb much of the incident light, so the EQE was less than 1%.
At first it wasn’t even clear that researchers would be able to extract the charge carriers from a thicker nanocrystal layer without losing most of them to electron–hole recombination. The usual wet-chemistry synthesis method produces nanocrystals capped with long-chain organic ligands. The long chains keep the nanocrystals from getting too close together in a close-packed array, and they hinder the mobility of electrons jumping from one nanocrystal to the next. Furthermore, the nanocrystals in a single batch aren’t all the same size, and the resulting mismatch in their electronic energy levels prevents charge transport via resonant tunneling.
The NREL researchers had found that by treating the nanocrystals with small organic molecules, they could replace the long-chain ligands with shorter ones, pack the nanocrystals more closely together, and improve the charge mobility. Choosing the best small molecule was a matter of trial and error. Ethanedithiol had worked in their earlier nanocrystal solar cells, but it reduced the MEG efficiency. Hydrazine maintained the MEG efficiency but compromised the solar cell’s performance. In the end they used a combination of the two chemicals. They’re still not sure how the electrons move between quantum dots of different sizes.
Efficiency peaks
To look for MEG, the researchers illuminated their solar cells with monochromatic light and measured the current output. Figure 2 shows the results for several of the best devices, most of which gave EQEs greater than 100% for some photon energies. The best EQE, 114%, was for a device with an antireflective coating. When they accounted for the reflected photons and those absorbed in inactive regions of the cells, they found IQEs of up to 130%.

Figure 2. The external quantum efficiency (EQE) of a solar cell is the ratio of collected charge-carrier pairs to incident photons. An EQE greater than 100%, seen here for several solar cells made with lead selenide nanocrystals, is definitive evidence of multiple exciton generation. The highest EQE, 114%, was for a cell with an antireflective (AR) coating. The other data are color-coded by bandgap for ease of viewing. (Adapted from ref.
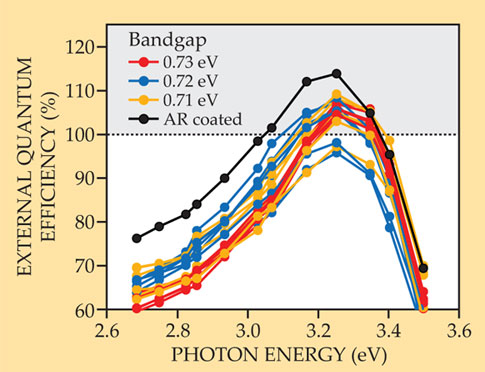
The peaks in figure 2 are for photon energies more than four times the nanocrystals’ bandgap, but the EQE values are nowhere near the thermodynamically allowed 400%. Some of the carrier pairs are lost to recombination, but the MEG itself is also not as efficient as it could be. By fitting their data to a model they’d developed, 6 the NREL researchers concluded that the MEG onset was around 2.6 times the bandgap. They’d like to drop that threshold closer to twice the bandgap (onsets as low as 2.1 times the bandgap have been reported for other materials) and to generate more carrier pairs per high-energy photon. Fortunately, according to their model, those two goals go hand in hand. Achieving them will require better theoretical understanding but also more empirical experimentation.
The MEG devices aren’t breaking any power-conversion records yet, and they’re nowhere near exceeding the Shockley–Queisser limit. Averaged over the solar spectrum, they convert about 4.5–5% of the incident light’s energy to electrical energy. (For comparison, the best single-junction bulk-material cells are more than 25% efficient, and the best nanocrystal-based cells are around 6% efficient.) One reason the power-conversion efficiency isn’t higher is that the charge carriers lose much of their energy on their way out of the cell—that is, the operating voltage is much less than the bandgap.
References
1. O. E. Semonin et al., Science 334, 1530 (2011). https://doi.org/10.1126/science.1209845
2. A. J. Nozik, Physica E 14, 115 (2002). https://doi.org/10.1016/S1386-9477(02)00374-0
3. L. R. Canfield et al., Metrologia 35, 329 (1998). https://doi.org/10.1088/0026-1394/35/4/19
4. R. D. Schaller, V. I. Klimov, Phys. Rev. Lett. 92, 186601 (2004). https://doi.org/10.1103/PhysRevLett.92.186601
5. J. B. Sambur, T. Novet, B. A. Parkinson, Science 330, 63 (2010). https://doi.org/10.1126/science.1191462
6. M. C. Beard et al., Nano Lett. 10, 3019 (2010). https://doi.org/10.1021/nl101490z
7. D. J. Binks, Phys. Chem. Chem. Phys. 13, 12693 (2011).https://doi.org/10.1039/c1cp20225a
More about the authors
Johanna L. Miller, jmiller@aip.org




