Monitoring surface diffusion, one molecule at a time
DOI: 10.1063/PT.3.1391
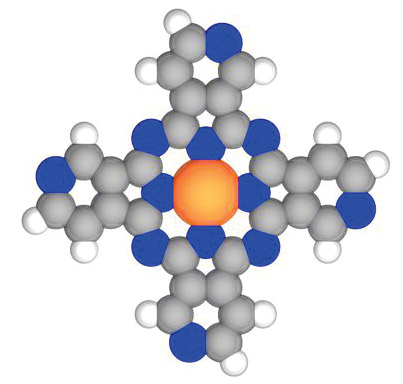
The symmetry of a surface can influence the preferred direction in which adsorbed molecules may diffuse—their motion constrained either to particular sites or to one dimension, for instance, because of surface anisotropy. But the converse is also true: An adsorbed molecule’s symmetry can also influence its diffusion—sometimes dramatically. A University of Regensburg group led by Jascha Repp has now studied that converse situation using a scanning tunneling microscope to image copper tetraaza phthalocyanine adsorbed on an ultrathin film of sodium chloride. The organometallic molecule, pictured here, forms four distinct isomers that differ only in the position of the nitrogen atoms (blue) localized on each arm of the molecule’s carbon framework. The STM resolved the orbital configuration, and thus the symmetry, of each isomer and its position on the NaCl surface. Crucially, the STM also was used to trigger an isomer’s movement by injecting enough current for it to overcome the diffusion barrier. The microscope’s tip was positioned atop a molecule at constant voltage until a drop in current occurred, signaling lateral motion. By performing that experiment repeatedly for distinct isomers, the Regensburg team found specific patterns of movement, which allowed them to determine the different symmetry-specific diffusion behavior. (T. Sonnleitner et al., Phys. Rev. Lett. 107, 186103, 2011.)