Metal–insulator transition in vanadium dioxide
DOI: 10.1063/1.4797176
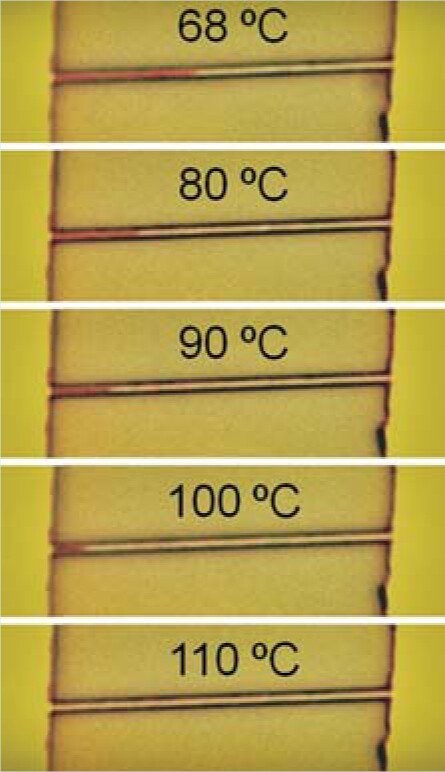
Below 68 °C vanadium dioxide is an insulator. Above that temperature it’s a metal. The nature of the transition has long remained elusive, though, because bulk VO2 has a domain structure that complicates its behavior. David Cobden and colleagues from the University of Washington have found an elegant way to avoid that difficulty and map the effective phase diagram. The team grew rectangular nanobeams that were thinner than the characteristic domain size of a few microns. They then suspended each beam between two electrical contacts. The metallic and insulating phases differ in lattice constant, but the constrained geometry creates a uniform stress field in a VO2 beam such that the two phases coexist in a range of temperatures between 68°C and 105°C. Thanks to the dramatic change in optical properties that accompanies the transition, Cobden’s team could visually track the nucleation and growth of the metallic phase as a function of temperature. The figure here shows five snapshots of a 20-µm-long beam (red indicates the insulating phase). By measuring the electrical resistance in the coexistence regime, the researchers found that the resistivity of the insulating phase is independent of temperature. That remarkable result, they argue, implies that the phase transition occurs at a fixed carrier density in the material and is consistent with a picture in which electron–electron interactions drive the transition. (J. Wei, Z. Wang, W. Chen, D. H. Cobden, Nat. Nanotech. 4 , 420, 2009 http://dx.doi.org/10.1038/nnano.2009.141