Mercury isotopes in Arctic snow exhibit mass-independent fractionation
DOI: 10.1063/1.3397033
Since industrialization, the amount of mercury circulating in the atmosphere has roughly tripled. But the physical and chemical processes involved in the Hg cycle are poorly understood. Without knowing where Hg goes once it’s emitted into the atmosphere—from a coal-burning power plant, say—or how long it remains actively cycling, it’s difficult to make informed decisions about Hg pollution.
One piece of information that can be used to track Hg through the cycle is its isotopic composition. The seven stable isotopes of Hg don’t behave identically in chemical reactions and physical processes. As a result, naturally occurring samples can have measurably different isotope ratios. In most observed isotope fractionation, deviations in reactivity depend on the mass difference between isotopes, due either to kinetic effects or to differences in the zero-point energy of chemical bonds.
In 2007 Bridget Bergquist and Joel Blum of the University of Michigan in Ann Arbor discovered a form of Hg isotope fractionation that breaks the mass-dependent pattern: Hg’s two stable magnetic isotopes, 199 Hg and 201 Hg, exhibit slightly different behavior from that of the five stable nonmagnetic isotopes. 1 Such mass-independent fractionation (MIF), seen in only a few elements so far, is thought to be due mostly to a coupling between nuclear and electronic spins that facilitates electron spin flips in certain photoinitiated reactions.
Bergquist and Blum made their discovery in a study of Hg in aquatic systems and fish tissues. Since then, Hg MIF has been observed in a variety of geochemical contexts. Now Blum, Laura Sherman, and colleagues have studied MIF in Arctic snow. 2 Their results suggest that Hg leaves the snow via a photochemical reaction that favors the odd-mass isotopes. And the unusual isotopic signatures they observed may be useful in identifying any Hg that enters Arctic ecosystems from the melted snow.
Falling mercury
Mercury is deposited in the snow seasonally: Shortly after the annual polar sunrise, sunlight triggers atmospheric mercury depletion events (AMDEs), in which much of the Hg in the polar atmosphere—an amount estimated at some 100 tons per year—binds to halogen atoms and precipitates. When the snow melts, any Hg remaining in it ends up in the water. But how and to what extent Hg leaves the snow by other means is uncertain.
The amount of Hg in Arctic snow can vary greatly from place to place, so monitoring the total amount from a few isolated measurements is nearly impossible. And the concentrations are too small—some tens to hundreds of nanograms per liter—for mass spectrometry to directly measure the parts-per-thousand differences in the relative amounts of 199 Hg and 201 Hg in the snow. So although Blum and colleagues collected snow samples from Barrow, Alaska, in March 2006, the isotopic analyses had to wait more than two years before team members Sherman and Kelsey Johnson developed a method for concentrating the Hg without inducing any additional isotope fractionation.
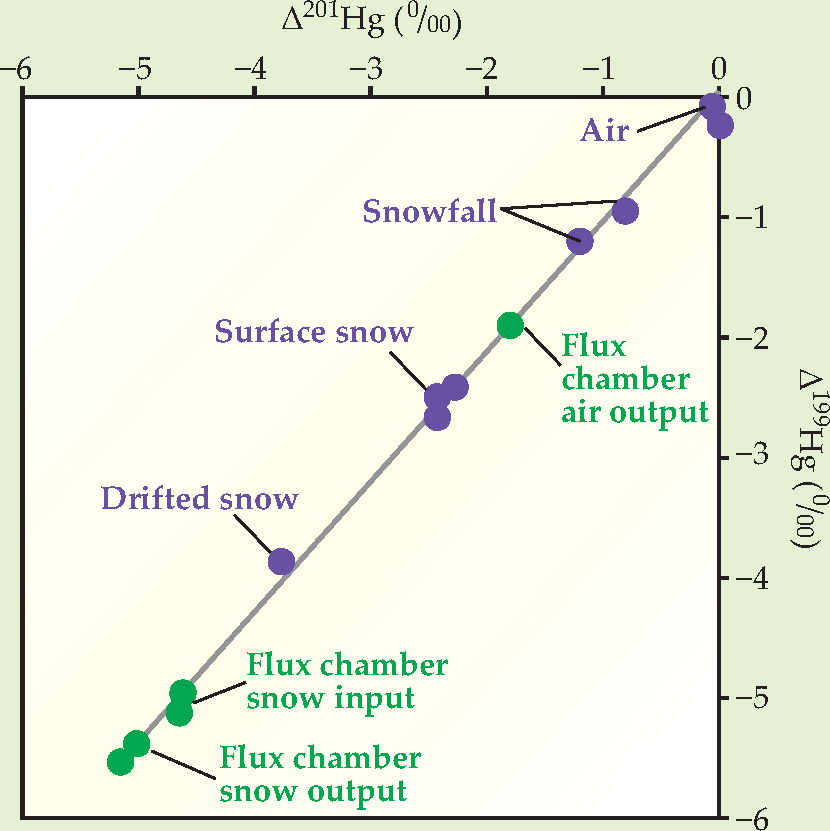
The purple dots in the figure show MIF measurements for several natural samples: Δ199 Hg and Δ201 Hg are the fractional differences between the measured amounts of each odd-mass isotope and the amounts that would be expected if the isotopic composition followed the mass-dependent pattern. Compared to the Arctic air, newly fallen snow collected in trays above the ground was depleted in 199 Hg and 201 Hg by about one part per thousand. Surface snow, collected several days after it fell, and drifted snow, which had been blowing across the tundra for an unknown but probably lengthy amount of time, contained even less of the odd-mass isotopes. Those measurements suggest that as the snow is exposed to sunlight, it loses Hg in a process that favors the magnetic isotopes and leaves more of the nonmagnetic ones behind.
To test that idea, the researchers performed a controlled experiment in the field, as shown by the green dots in the figure. They filled a so-called flux chamber with snow (they used drifted snow, which already had a negative MIF) and passed ambient air through the chamber while exposing it to the Arctic sunlight. They found that after 10.5 hours of sun exposure, the Hg remaining in the snow contained even less 199 Hg and 201 Hg, whereas the Hg transferred to the air had more.
That the observed MIF was always negative—that the odd-mass isotopes were more reactive—came as a surprise. Current understanding is that MIF occurs when light excites a molecule, such as one of the Hg-halogen molecules created during an AMDE, to create a so-called radical pair, two unpaired electrons of parallel spin. Coupling with a magnetic nucleus can induce one of the electron spins to flip and the molecule to return to its original state. But that mechanism should make the magnetic isotopes less reactive, not more. The researchers speculate that Hg could be emitted from the snow by a more complicated, multistep reaction in which the spin flips somehow enhance rather than diminish reactivity. They also stress that the MIF mechanism itself may not be fully understood. Says Sherman, “I would not be surprised if future studies show that the current view is overly simplistic.”

Mass-independent mercury fractionation measurements of Arctic air and snow samples (purple) and a flux-chamber experiment (green). Together, the results indicate that exposure to sunlight decreases the amounts of the magnetic isotopes 199Hg and 201Hg in a snow sample.
(Adapted from ref. 2.)
References
1. B. A. Bergquist, J. D. Blum, Science 318, 417 (2007). https://doi.org/10.1126/science.1148050
2. L. S. Sherman et al., Nat. Geosci. 3, 173 (2010).
More about the authors
Johanna L. Miller, jmiller@aip.org




