Mechanically stressed phytoplankton light up
DOI: 10.1063/PT.3.4564
English naturalist Henry Baker in 1753 identified the source of the phosphorescent “burning of the seas,” like the glow off the Taiwanese coast in figure
Figure 1.

Swarms of the dinoflagellate Noctiluca scintillans emit blue light that illuminates the coastline in Taiwan. In historical accounts of nautical adventures, mariners marveled at the milky seas caused by bioluminescence. (Photo by RobbinChang.)

The complex series of chemical reactions through which bioluminescent marine organisms produce light begins when calcium ions enter the cell through channels in the cell wall. Biologists hypothesize that fluid flow outside the cell causes the cell’s outer wall to stretch, which opens those ion channels. One puzzle, however, arises from experiments that use different methods of stimulating the cell to elicit a bioluminescent flash: How exactly do those methods, which involve both fluid and mechanical perturbation, lead to a distribution of forces over the cell body that cause the cell to stretch?
Maziyar Jalaal, Raymond Goldstein, and colleagues at the University of Cambridge now have taken a step toward solving that puzzle. In a series of experiments, the researchers pinpoint the mechanical stresses that deform the viscoelastic membrane of the dinoflagellate Pyrocystis lunula and trigger light emission. 1 Linking macroscopic flows to microscopic cellular processes could improve understanding of the ecology and evolution of bioluminescent organisms.
Poking and prodding
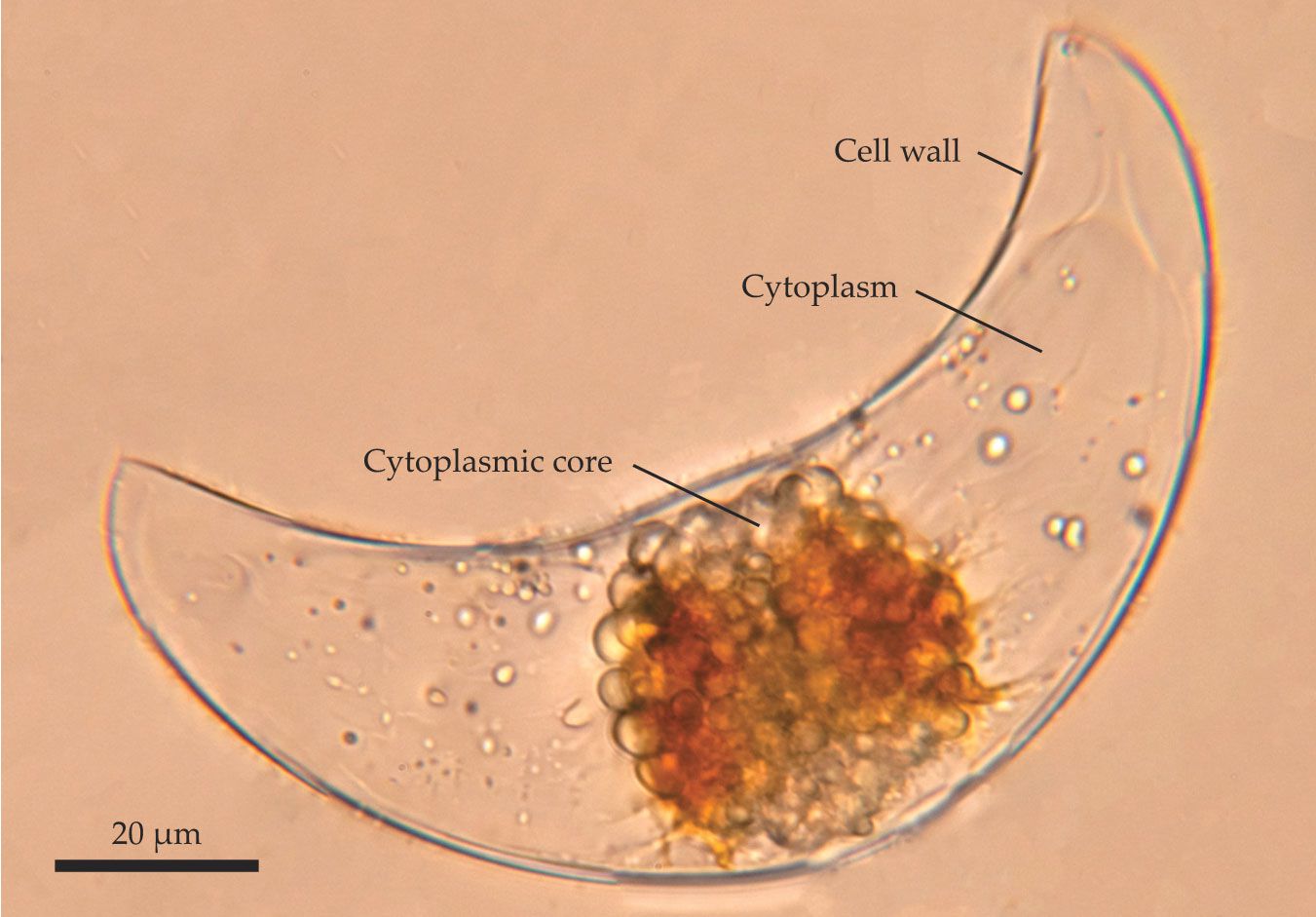
P. lunula has occupied the spotlight for bioluminescence studies for several decades. The large (130 μm long) organism, shown in figure
Figure 2.

A single cell lights up. The unicellular marine phytoplankton Pyrocystis lunula is a common subject for bioluminescence studies. The organism’s cytoplasmic core emits flashes of light when external stresses push on the cell wall. (Adapted from ref.
Subsequent studies using improved flow visualization tools have shed light on the process. In the early 2000s, Michael Latz and colleagues at the Scripps Institution of Oceanography estimated the magnitude of stress required to trigger light production in P. lunula under different methods of forcing. In one study, they poked a cell at a single point using an atomic force microscope until the cell lit up. 3 In other studies, they pumped water alongside the cell and found that emitted light intensity depends on the shear. 4 And zoophysiology experiments performed by Latz’s group have shown how much stress is required to trigger stretch-activated proteins in dinoflagellate bioluminescence signalling pathways. 5
But there are discrepancies in bioluminescent responses to different forms of stimulation. Those variations are likely based on the unknown distribution of sensors in the cell that respond to mechanical stimulation and on how the sensors’ activity is integrated into a whole-cell response.
Jalaal and his colleagues have now performed a quantitative study that could help resolve the discrepancies. Their experiments explored two ways of stimulating P. lunula cells to flash. In the first protocol, the researchers pumped a liquid through a microscopic pipette to create a shearing force along the surface of a cell (figure
Figure 3.

Two different mechanisms trigger a Pyrocystis lunula specimen to produce light. In both experiments, the unicellular phytoplankton sits on the tip of a micropipette. (a) Under fluid stimulation, a jet of liquid directed toward the cell at velocity u creates normal and shear forces. The upper half sketches the flow lines (blue), and the color map indicates flow speed u; the lower half shows streaks of particle tracers in the fluid. (b) Streamlines map out the flows that ultimately deform the cell wall (red) and elicit light production. (c) Under direct mechanical stimulation, a second pipette squeezes the cell. (d) The pipette deforms the cell wall (red) by an amount δ until light production ceases. (Adapted from ref.
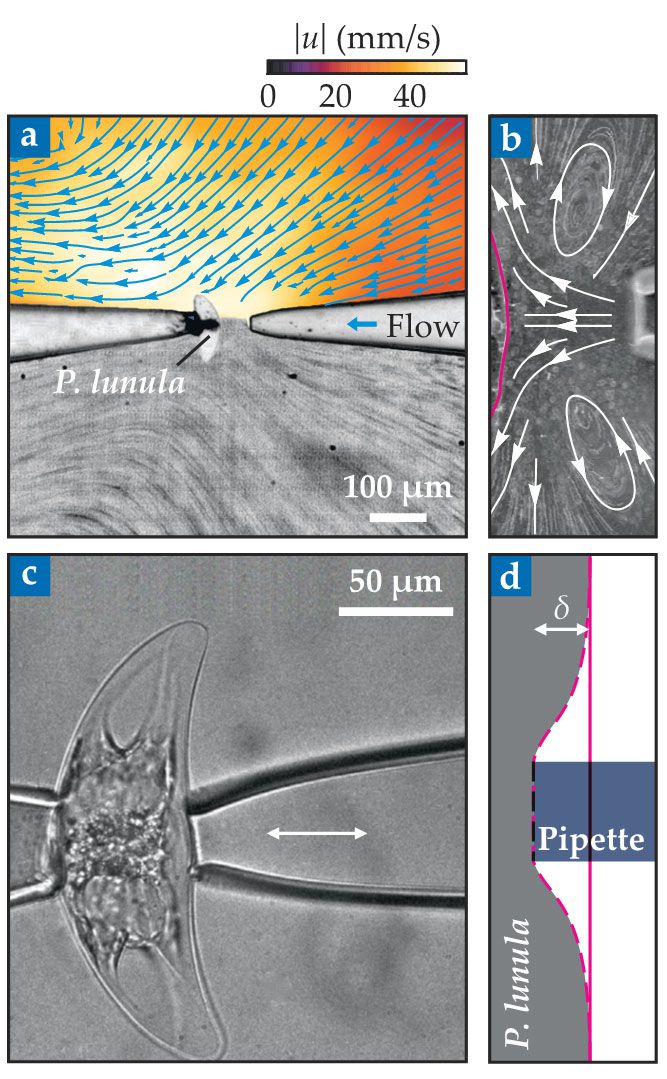
In the second protocol, the researchers used two pipettes to hold and gently squeeze the cell, and that squeezing created a highly localized deformation (figure
Sensitive stretches
For both experimental protocols, the imposed forces were increased at a constant rate to achieve a final deformation, after which the force was held fixed. P. lunula emitted a quick flash of light that slowly decayed. Observations of light intensity at different deformation rates (10–900 μm/s) and magnitudes (1–10 μm) showed that light intensity depends not only on how much the cell is deformed but also on the speed of the deformation. By repeating both experiments several times on individual cells, the researchers showed that the maximum light intensity emitted by a cell decreased in subsequent perturbations.
The findings led Jalaal and coworkers to develop a mathematical model that quantifies light production as a viscoelastic response. The model describes the triggering signal in terms of a strain rate that’s scaled by the relaxation time of the viscoelastic cell wall. The relationship between that signal and the emitted light intensity depends on the time scale on which the light-emitting biochemical reaction completes and on the time scale on which the cell resets and prepares to flash again. The triggering signal saturates at a maximum value—perhaps when all available ion channels in the cell membrane are open: The signal might be a proxy for the influx of calcium that results from the opening of channels. The model captures the observation that peak light intensity occurs when cells are highly deformed at high speed.
Combined with earlier studies that involved perturbing cells with different mechanical forces or flows, the new observations suggest that a cell may generate the same amount of light in response to a small force spread over a large area or a large force focused on a small area—that is, the light could result from weakly stretching many ion channels in the cell membrane or from strongly stretching just a few. Jalaal notes that further studies will be needed to investigate more realistic environmental fluid-flow conditions and determine “the distribution of forces on the cells and details of fluid-cell membrane interaction.”
Bioluminescence is a tool that organisms use to communicate with their surroundings—for example, to scare off predators. An improved understanding of its biochemical and physical origins provides the opportunity to explore its ecological significance across scales and species.
References
1. M. Jalaal et al., Phys. Rev. Lett. 125, 028102 (2020). https://doi.org/10.1103/PhysRevLett.125.028102
2. W. H. Biggley et al., J. Gen. Physiol. 54, 96 (1969). https://doi.org/10.1085/jgp.54.1.96
3. B. Tesson, M. I. Latz, Biophys. J. 108, 1341 (2015). https://doi.org/10.1016/j.bpj.2015.02.009
4. P. von Dassow, R. N. Bearon, M. I. Latz, Limnol. Oceanogr. 50, 607 (2005). https://doi.org/10.4319/lo.2005.50.2.0607
5. K. Jin et al., J. Phycol. 49, 733 (2013). https://doi.org/10.1111/jpy.12084




