Mechanical rupture explains a bacterium’s puzzling “pop”
DOI: 10.1063/PT.3.2835
Staphylococcus bacteria undergo cell division roughly once every 30 minutes. But when the time comes, the actual splitting of one cell into two happens in less than the blink of an eye—about a millisecond, to be precise. The event, known as “popping,” is too swift to be explained by the enzyme-mediated processes thought to govern conventional cell division. Now Julie Theriot and coworkers at Stanford University have made the case that popping is driven not by biochemistry but by mechanics. 1
As a Staphylococcus cell prepares to divide, it constructs a double-layered septum that partitions the spherical cell into two hemispheres—the soon-to-be daughter cells. Theriot and her colleagues postulate that as the cell continues to grow, stress builds along the peripheral ring where the septum meets the cell wall until, eventually, the ring cracks. As the crack grows in both directions around the cell’s circumference, the daughter cells tear away from one another, each taking a layer of the septum with it. Based on previous measurements of crack-propagation speeds in synthetic analogues of cell walls, 2 such a rupture could easily account for popping’s short time scale.
Although the popping events happen too quickly for the Stanford team to image them in detail, Theriot’s graduate student Xiaoxue Zhou was able to use a fluorescence imaging technique known as structured illumination microscopy to investigate how the wall of a parent cell evolves during the division process. She labeled a population of Staphylococcus aureus cells with wheat germ agglutinin, a fluorescent protein that binds to the cell’s wall but can’t penetrate it, and then imaged 40 popping events.
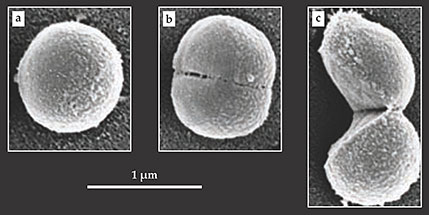
After each event, the old cell wall appeared to have hinged open like a clam shell, consistent with division mediated by a single propagating crack. In all but one case, the two halves remained momentarily connected at the hinge point. Scanning electron microscope images such as those shown in the figure below painted a similar picture of S. aureus’s division process. It’s likely that the other members of the Staphylococcus genus divide in the same manner.
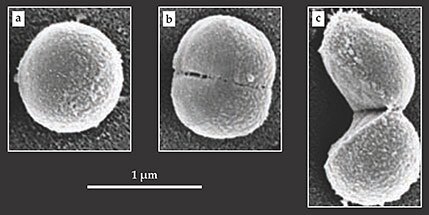
Scanning electron microscope images capture Staphylococcus aureus cells (a) well before, (b) just before, and (c) just after division. The bacterium’s split is swift; it takes only a millisecond or so for a single cell to separate into two. That process, known as popping, is thought to be mediated by a stress-induced crack in the cell wall. (Adapted from ref.
Enrique Rojas, a postdoc in Theriot’s lab, captured further experimental evidence of popping’s mechanical origins: He found that he could induce popping in a colony of S. aureus by suddenly diluting its growth medium. Such dilution elicits a sharp jump in the cells’ turgor pressure, the outward pressure on the cell walls, as the bacteria take up water to restore their osmotic balance. The manipulation also worked the other way around; Rojas could suppress popping by rapidly concentrating the growth medium.
Stretching and necking
At tens of nanometers, the walls of Staphylococcus cells are thick by microbial standards. It’s unlikely that turgor pressure alone would cause them to tear. The figure’s panel b, which shows a cell on the verge of popping, hints at a possible explanation. It reveals nanometer-sized perforations along the ring where the cell will eventually split. Presumably, those weak spots serve as potential nucleation sites for cracks. Might the perforations be the work of enzymes?
According to the Stanford group, probably not. Although Staphylococcus cells often use hydrolase enzymes to break down and remodel their cell walls, the researchers found that mutants lacking atl—the gene that encodes the bacterium’s main hydrolase—popped at the same rates and exhibited the same pattern of perforations as cells that had the gene.
The researchers think the perforations are more likely attributable to necking, a generic mechanical instability in which a band of ductile material, stretched taut, thins and weakens at random, localized regions along its length. The team’s numerical simulations suggest that during the late stages of a Staphylococcus cell’s division cycle, the tension along the ring joining the septum with the cell wall may indeed be great enough for the instability to kick in.
The proposed mechanism could help to explain another of Staphylococcus’s morphological mysteries—the tendency of its cells to cluster not into neat arrays but into irregular, grape-like bunches. 3 Because the location and orientation of the hinge axis is random from cell to cell, so are the relative arrangements of each daughter-cell pair.
Cell division by mechanical rupture may not be unique to Staphylococcus. “We looked at Micrococcus, and they seem to pop as well,” Theriot says. “If you talk to people who study cerevisiae, a budding yeast, they all talk about a point in cell division called cracking, where the bud cracks off. Mycobacterium, which causes tuberculosis, does something very similar.”
Theriot sees those and other examples as a sort of cautionary tale: “There’s often a starting assumption that complex biological processes—such as a cell deciding when and where to divide—must involve genes and proteins, so we often try to inhibit unwanted processes by targeting the genome.” But for mechanically driven phenomena such as popping, she says, that approach wouldn’t necessarily work. “In these cases, the cell is simply taking advantage of the fact that it’s a material object. It’s just obeying the laws of physics.”
References
1. X. Zhou et al., Science 348, 574 (2015). https://doi.org/10.1126/science.aaa1511
2. T. Baumberger, C. Caroli, D. Martina, Nat. Mater. 5, 552 (2006). https://doi.org/10.1038/nmat1666
3. M. G. Pinho, M. Kjos, J.-W. Veening, Nat. Rev. Microbiol. 11, 601 (2013).https://doi.org/10.1038/nrmicro3088




