Magnetic fields facilitate water electrolysis in microgravity
DOI: 10.1063/pt.awob.hsgu

Figure 1. The Bremen Drop Tower is a unique facility for microgravity research. In the tower’s unobstructed interior, experiments can experience free fall for more than 9 seconds. Researchers have used the facility to show how magnets can separate gas bubbles from water in space. (Photo © ZARM/University of Bremen.)

Bremen, Germany, is not known for its skyscrapers. The tallest structure in the city is a 236 m telecommunications tower. The second tallest, the 146 m Bremen Drop Tower (shown in figure 1
Diverse physical phenomena are studied at the Bremen Drop Tower, including turbulent flow and matter-wave interferometry. But the center’s codirector Katharina Brinkert is an electrochemist with an interest in developing systems to produce oxygen and fuels, such as hydrogen, for space exploration missions.
On Earth, the electrochemical splitting of water into its component elements is a familiar and well-studied reaction. Hydrogen accumulates at the cathode, oxygen at the anode, and both gases bubble up through the liquid and can be collected at the surface. But without gravity, there is no “bubbling up.” Some other forces are needed to pull the gas bubbles away from the electrodes.
Now Brinkert has teamed up with ZARM research associate Ömer Akay, Georgia Tech assistant professor in aerospace engineering Álvaro Romero-Calvo, and others to show that magnetic fields might do the job in not just one but two ways. 1 In their proof-of-principle experiments at the Bremen Drop Tower, the researchers demonstrated two fundamentally different uses of magnetic forces to facilitate water electrolysis in microgravity. In one, the forces act on the water molecules themselves; in the other, they act on ions in solution.
Lighter load
Magnetism isn’t the only way to separate bubbles from water in microgravity, and it’s not the first thing that’s been tried. The life-support system on the International Space Station uses water electrolysis to produce oxygen for the astronauts to breathe—water is easier and safer than oxygen to carry in large amounts—and it uses a centrifuge to separate the reaction products. But the machinery uses a lot of energy, and its moving parts are prone to failure.
For a space mission, mechanical unreliability introduces a unique set of practical challenges, as Romero-Calvo explains. “Imagine you want to take a road trip across the desert,” he says. “There are no service stations. What do you do if you get a flat tire? Well, you have to bring another tire with you—and not just one, but as many as you think you’ll need for the whole trip.”
There are no spacecraft repair shops in space. Astronauts need to bring with them all the spare parts they’ll need for the whole mission, or at least until their next contact with Earth. For long missions, such as a future crewed trip to Mars, the mass of the payload adds up quickly, and so does the expense.
In that context, magnets have the considerable advantage that there’s nothing to break. Neodymium permanent magnets produce extraordinarily strong magnetic fields, but with no moving parts and no power consumption. They should, in principle, reliably last for as long as they’re needed.
What goes up
Water electrolysis under magnetic fields has been studied on Earth. “But the beauty of space research is that you can see lots of cool effects that are usually masked by gravity,” says Romero-Calvo. The results aren’t always what one expects, he explains, and there’s no substitute for actually doing the experiments.
To bring microgravity to Earth, the Bremen Drop Tower exploits the well-known principle that when a whole system is in free fall, gravity acts on all parts of it equally, and it behaves as if there were no gravity. But the facility takes it a step further. Instead of carrying experimental devices to the top of the tower and dropping them, which would give a free-fall time of about 4.7 seconds, ZARM researchers launch their setups upward from ground level. The systems are subject to strong forces upon liftoff (and upon landing), but for the rest of their 9.3-second trajectory—on the way up and the way down—the devices experience weightlessness.

Figure 2. Diamagnetic repulsion pushes water away from magnets. So when a pair of magnets flank an electrochemical cell, the magnetic field B pushes the water toward the electrodes at the center. There, it displaces the hydrogen and oxygen gas bubbles, which flock toward the magnets. (Images adapted from Ö. Akay et al.
“There’s nothing like it in the US,” says Romero-Calvo. In comparison, NASA’s Zero Gravity Research Facility in Cleveland, Ohio, has an underground drop shaft that’s slightly taller than the Bremen Drop Tower, but it’s equipped only for one-way trips of 5.2 seconds.
How do they work?
Water is diamagnetic, which means that its molecules are repelled by magnets. Normally, the force of repulsion is far too weak to have any practical effect unless the magnet is extremely strong. It was diamagnetism—mostly of water—that allowed Andre Geim and colleagues, in 1997, to famously levitate a frog. (See the article by Geim in Physics Today, September 1998, page 36
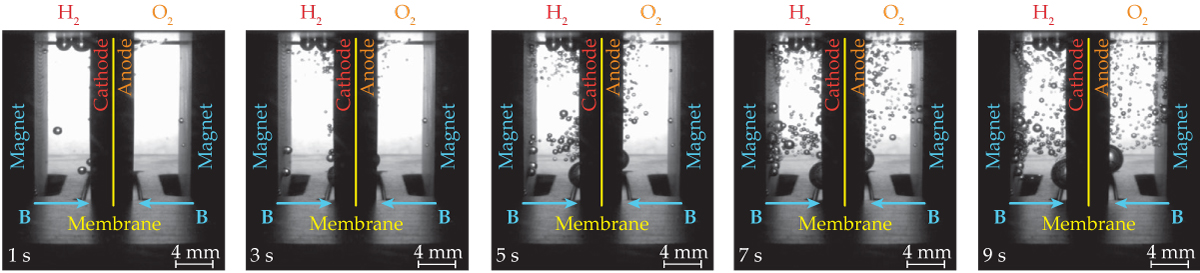
Levitating a frog in Earth’s gravity required a 20 T electromagnet. The Nd permanent magnets used in the water-electrolysis experiments have just a few percent of that strength. But without the force of gravity to overcome, even a modest diamagnetic repulsion can be significant. The results of a 9-second ZARM test are shown in figure 2
The electrochemical cell used in the first round of ZARM tests is about 1 cm in width. When an electric current is applied to a pair of mesh electrodes, bubbles of H2 and O2 quickly form on either side of a proton-exchange membrane. Without magnets, the gases would build up on the electrodes, and the reaction would grind to a halt. But magnets at the cell’s outer edges push the water toward the cell’s center. The bubbles, as a result, flow away from the electrodes and toward the magnets.
The team’s second setup was cinematically inspired. In The Hunt for Red October, Sean Connery’s character’s submarine is powered by a magnetohydrodynamic drive: Seawater is subjected to both an electric current and a magnetic field, and the Lorentz force acting on the salt ions sends the water spiraling in a corkscrew trajectory that pushes the sub forward. Reality is not quite as elegant as fiction, but when the researchers designed a cell with ring-shaped electrodes and placed it in a magnetic field, the Lorentz forces did work to sweep the bubbles off the electrodes.
All coming together
“What we demonstrated is a TRL-3 technology,” says Romero-Calvo, referring to NASA’s scale of technology readiness levels: TRL 3 refers to the lab demonstration of a technology’s critical function. “To get this to orbit,” he says, “we’ll need to reach TRL 8,” with a full-scale electrolysis system built, tested, and ready to go.
In addition to constructing a larger electrolysis cell and testing it for more than 9 seconds, the researchers will need to grapple with several more practical considerations. For example, how will the product gases be collected from the cell, and how much humidity and other impurities will they contain? How much energy will the electrolysis consume, and will it be possible to integrate the cell directly with solar-powered photoelectrodes? With ongoing funding from NASA and the European Space Agency, the researchers are already working on those next steps.
The physics of magnetism at the heart of the technology is old news. If it’s such an appealing way to separate liquids from gases in microgravity, why hasn’t it been considered before now? “That’s a good question,” says Romero-Calvo. “I believe it’s because we put together so many concepts from distant fields. Space electrochemistry is a very small field. Magnetic electrolysis is another very small field. Sustained access to microgravity is even more exceptional. The fundamental-science and applied-engineering perspectives are hard to mix. It’s quite an exceptional combination of factors that really enabled our work.”
References
1. Ö. Akay et al., Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01890-0




