Isotope ratios hint at a piece of pristine Earth
DOI: 10.1063/1.3502539
A few humans have been from the Earth to the Moon, and many more have been around the world in 80 days or fewer. But no one has yet made a journey to the center of the Earth. Our experience with the planet on which we live is almost entirely confined to the material and information that makes its way to the surface.
For that reason and others, precise measurements of Earth’s overall composition are difficult or impossible. The continental crust, the most familiar part of Earth for most of us, is not a representative sample: Its composition is not even the same as that of the crust beneath the oceans. The difference is attributed to the effects of partial melting of the silicate mantle that lies beneath the crust. Certain incompatible elements—so called because they strain the crystal lattices of the solid mantle—were preferentially pushed out of the solid and into the melt. The continents formed from the melt; the oceanic crust formed, and is constantly regenerated, from the material left behind in the upper mantle, depleted in the incompatible elements. Since then, volcanism, tectonic-plate movements, and thermal convection have all served to transport material within and across the crust and mantle, slowly stirring the silicate portion of Earth like a batch of cookie dough.
Geochemists have long assumed, quite reasonably, that Earth as a whole has the same composition as the rest of the solar system, best represented by certain meteorites called chondrites. The chondrites never underwent the large-scale differentiation that Earth did, so it’s easy to measure their compositions. Their isotopic compositions, in particular, yield important clues about long-gone radioactive elements and their abundances on Earth.
In 2005, however, Richard Carlson and his postdoc Maud Boyet made a surprising discovery
1
(see also Physics Today, September 2005, page 19
Now, partially based on that discovery, Matthew Jackson—another of Carlson’s postdocs, now at Boston University —and collaborators have found that rocks from Baffin Island in northeastern Canada, the products of a massive volcanic eruption 60 million years ago, show all the signs of having come from an ancient mantle reservoir, undifferentiated and unmixed since not long after Earth’s formation 4.5 billion years ago. 2 Whether Earth as a whole is Sm rich or not, the Baffin samples appear to be made of the original Sm-rich material from which all the continental and oceanic crust is derived. The researchers base their conclusion on iso-topic measurements of several elements, including Nd, helium, and lead.
Helium
Earth’s primordial helium (primordial with respect to the formation of the solar system, not the universe) contained considerably more 3He than is present in the air today. Helium in the air is eventually lost into space. It’s replaced by radiogenic He, which is almost exclusively 4He produced in α decays. (Neutron capture by lithium-6 yields some 3He, but the amount is negligible for most purposes.)
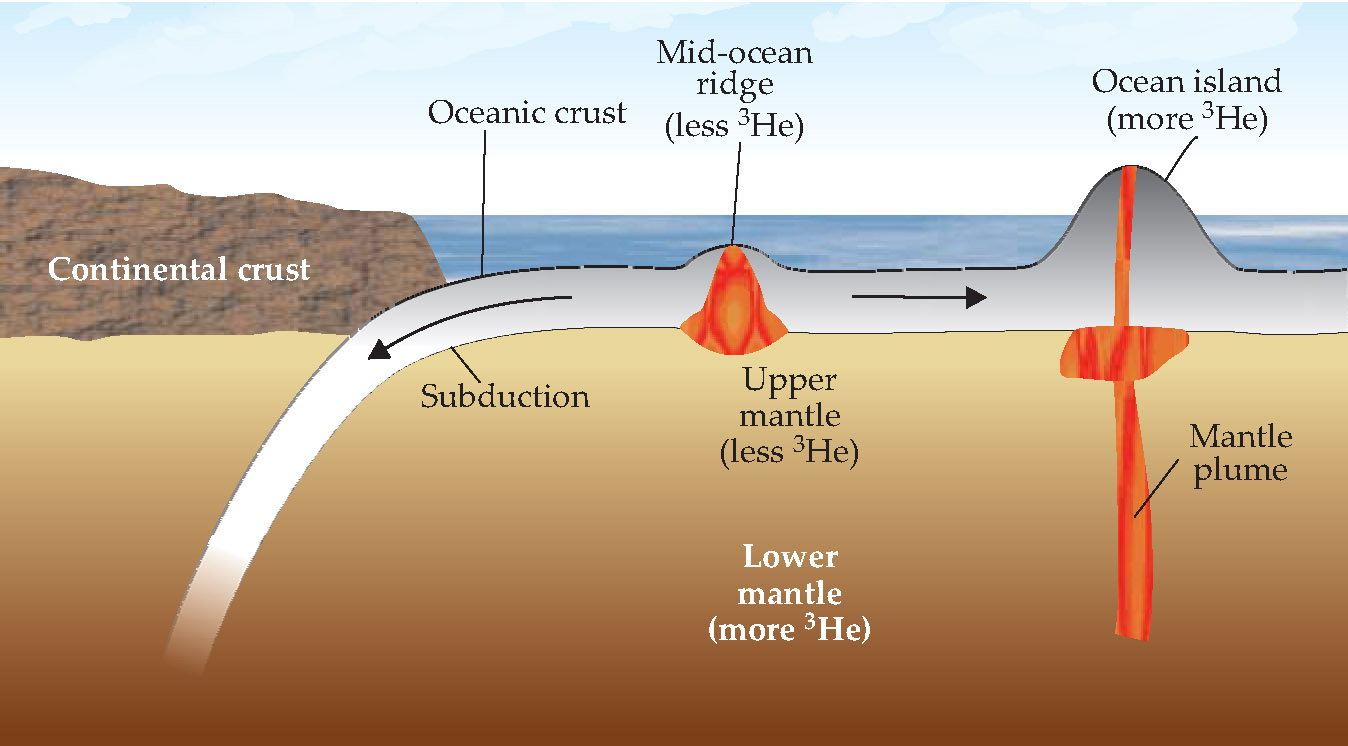
Much of Earth’s He, primordial and radiogenic, remains trapped in the crust and mantle. When mantle material melts and reaches the surface, it degases, losing much of its He to the atmosphere. Higher 3He/4He ratios should therefore signal material that’s remained relatively unexposed to the surface over geologic time. Indeed, the 3He/4He ratio is low for mid-ocean ridge minerals, which form from the mantle just beneath the crust, as shown in figure 1. The ratio is often higher on ocean islands, which form from plumes originating in the lower mantle.

Figure 1. As oceanic crustal plates pull apart at the mid-ocean ridges, upper-mantle material melts and wells up to create new crust. The old crust subducts, or sinks beneath a neighboring plate back into the mantle. Ocean islands, in contrast to the ridges, form from molten plumes originating deep within the mantle. In each process, the molten mantle gives up some of its helium, including primordial 3He that is never replaced. The upper mantle is thought to be more processed and more degassed than the lower mantle, so the mid-ocean ridges contain less 3He than the ocean islands.
Samples from several islands, including Hawaii, Iceland, Samoa, and the Galapagos, have been found to have especially high 3He/4He, more than 30 times that of air. But, as measured in 2003 by Finlay Stuart and colleagues, the Baffin Island rocks have the highest ratio ever found on Earth: up to 50 times that of air. 3 Those researchers concluded that the Baffin material was not from a pristine mantle reservoir, however, in part because of their Nd measurements.
Neodymium
Neodymium-142, the subject of Boyet and Carlson’s 2005 investigation, is not normally a useful probe of a sample’s geological history. Any place-to-place variation in the 142Nd/144Nd ratio would have to be caused by partial separation of 144Nd and 146Sm, 142Nd’s parent. Since the two Nd isotopes, both stable, behave virtually identically, the 142Nd/144Nd ratio has been locked in place ever since all the 146Sm had decayed into 142Nd, a few hundred million years after Earth’s formation. Indeed, all of Boyet and Carlson’s terrestrial samples had the same 142Nd/144Nd ratio to within a few parts per million, the resolution of their measurement.
But Nd has another radiogenic isotope, 143Nd, the daughter of the much longer-lived 147Sm. Since much of Earth’s original 147Sm is still around, geological processes over the whole of Earth’s history can and do separate 147Sm and 144Nd, yielding measurably different 143Nd/144Nd ratios over a much larger range of about one part per thousand.
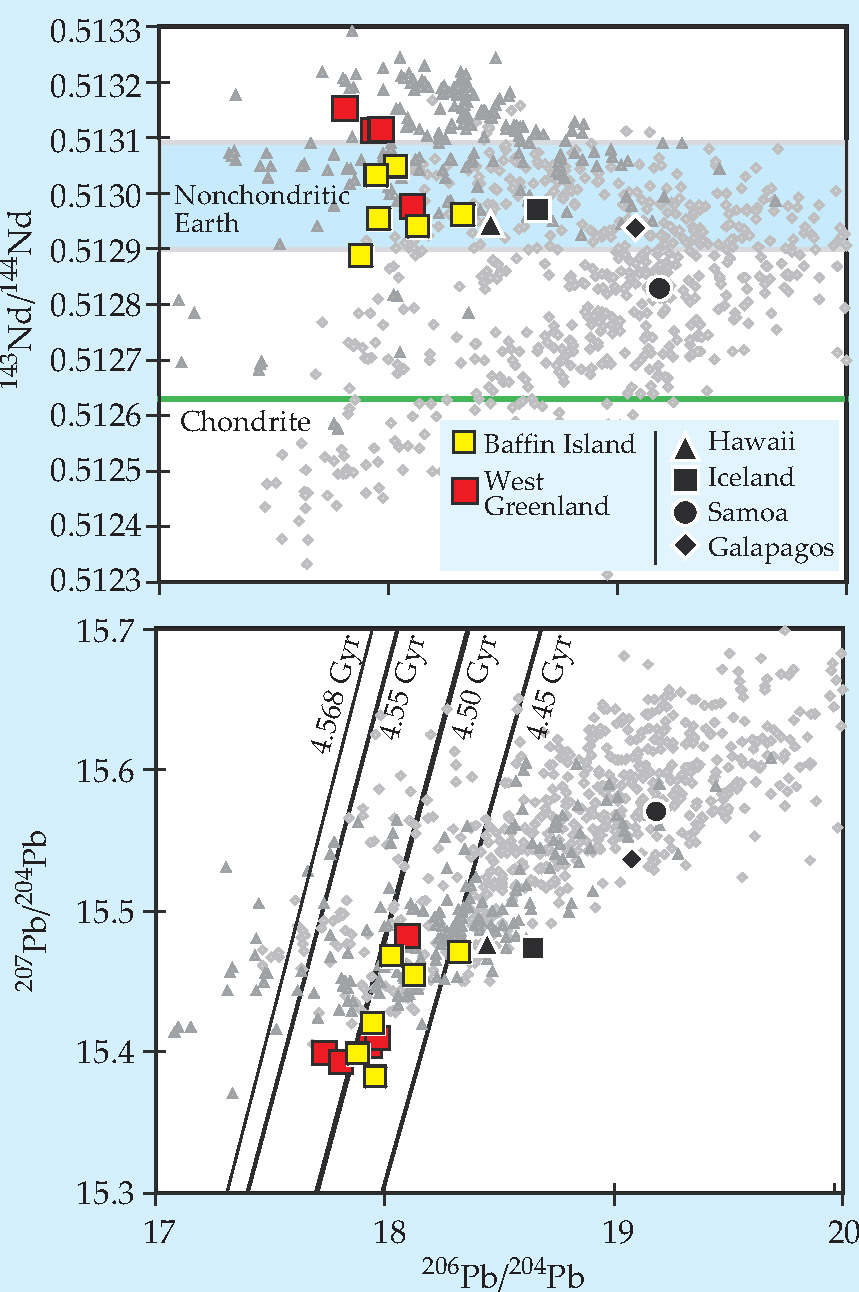
If Earth’s composition were chondritic, then primitive, undifferentiated mantle material would have a 143Nd/144Nd ratio of about 0.5126, the same as that of the chondrites. But since, as Boyet and Carlson found, the accessible part of Earth formed with more 146Sm than the chondrites, it must also have had more 147Sm. The expected 143Nd/144Nd for primitive terrestrial mantle thus works out to between 0.5129 and 0.5131.
As shown in the top panel of figure 2, the rocks from Baffin Island and from neighboring West Greenland do fall within the 0.5129-0.5131 range. When Stuart and colleagues measured those values in 2003, they inferred not that the samples were from a primitive reservoir but that they were mixtures of material from two different parts of the mantle, neither of which represented a primitive composition.

Figure 2. Isotopic measurements of Baffin Island (yellow), neighboring West Greenland (red), other especially helium-3-rich ocean islands (black), and other terrestrial samples (gray). As the top panel shows, the neodymium compositions of the Baffin samples fall within the range (blue stripe) expected of primitive material in a nonchondritic Earth. As the bottom panel shows, their lead isotopic compositions cluster around the 4.50-billion-year isochron, as expected of a mantle reservoir that’s been isolated since early in Earth’s history.
(Adapted from
Lead
It was Jackson’s idea to revisit the Baffin Island samples in light of Boyet and Carlson’s 142Nd results, by looking at Pb. Like Sm decaying into Nd, two particular isotopes of uranium decay into two isotopes of Pb through a series of α and β decays: 235U into 207 Pb and 238U into 206 Pb. All the intermediate elements in each decay chain have short half-lives, so the decay rate is governed by the U half-life: 700 million years for 235U and 4.5 billion years for 238U.
Unlike 146Sm, neither U isotope has entirely decayed away, so the amounts of both radiogenic Pb isotopes, measured with respect to the nonradiogenic 204 Pb, can vary considerably from sample to sample. The key to making sense of the measurements is to realize that since the two U isotopes decay at different rates but otherwise behave almost identically, the 235U/238U ratio is a function of time but not of position. It follows that on a plot of 207Pb/204 Pb versus 206Pb/204 Pb, a system that’s been isolated for a time t will fall somewhere on a line, called an isochron, whose location depends on t. Where on the line it falls depends on the U/Pb ratio that the system started with.
As shown in the lower panel of figure 2, the Baffin and West Greenland samples are clustered around the 4.50-billion-year isochron, as expected for material that’s been isolated since shortly after Earth’s mantle formed. All of the other high-3He samples fall well to the Baffin samples’ right. That doesn’t mean that their ages are given by the isochrons through those points, rather that at some point in their history—possibly during their journey through the mantle to the surface—they mixed with material of different composition.
As the world churns
If the Baffin samples are indeed from an ancient mantle reservoir, how could any part of the mantle have remained undifferentiated for so long? Many geo-scientists once thought that the answer was easy. Studies of seismic-wave speeds reveal a pressure-induced phase boundary at a depth of 660 km; that boundary, it was thought, could be a barrier to mixing. If the upper and lower parts of the mantle never mixed, then the lower mantle would have remained pristine.
Subsequent seismic studies, however, have shown that picture to be far too simple. As new oceanic crust is created at the mid-ocean ridges, the old crust sinks, or subducts, beneath the neighboring tectonic plate and into the mantle, as shown in figure 1. The slabs of subducted crust appear to extend far past the 660-km discontinuity almost all the way to the core-mantle boundary. (See Physics Today, August 1997, page 17
But it can’t be mixed to the point of homogeneity. Even setting aside Jackson and colleagues’ new work, the 3He/4He measurements make it clear that the mantle’s composition is not uniform. The challenge for geophysical modelers is to reconcile the seismic measurements with the isotopic ones. Some models show every part of the mantle affected by mixing, but not uniformly. 4 They could explain the high 3He/4He ratios, but not the preservation of any primitive material.
Other models show primitive material preserved in pockets. For example, if the subducted crust is more than a few percent denser than the surrounding mantle, it sinks to and accumulates around the core. 5 As the dense pools build up, they could trap blobs of uncontaminated mantle material, possibly for billions of years.
References
1. M. Boyet, R. W. Carlson, Science 309, 576 (2005). https://doi.org/10.1126/science.1113634
2. M. G. Jackson et al., Nature 466, 853 (2010). https://doi.org/10.1038/nature09287
3. F. M. Stuart et al., Nature 424, 57 (2003). https://doi.org/10.1038/nature01711
4. H. M. Gonnermann, S. Mukhopadhyay, Nature 459, 560 (2009). https://doi.org/10.1038/nature08018
5. J. P. Brandenburg et al., Earth Planet. Sci. Lett. 276, 1 (2008). https://doi.org/10.1016/j.epsl.2008.08.027
More about the authors
Johanna L. Miller, jmiller@aip.org




