Interferometry data challenge prevailing view of wave propagation in the cochlea
DOI: 10.1063/1.2911168
So sensitive is the human ear that sound pressures light enough to vibrate the eardrum by mere tenths of an angstrom generate waves that are amplified in the cochlea and then passed on to the brain as electrical signals. Although details are murky at the cellular level, the basic physics of hearing is straightforward: Vibrational motion of the stapes bone in the middle ear creates a pressure difference across the basilar membrane, an elastic ribbon that partitions the fluid-filled cochlea lengthwise into two chambers. The pressure difference creates a slow traveling wave along the membrane, whose elasticity acts as a restoring force.
The membrane’s width increases along the cochlear spiral, from base to apex. The change in width is accompanied by a decrease in its stiffness, which makes the cochlea a frequency analyzer: Regions nearer its base resonate with higher-frequency signals; regions closer to its apex resonate with lower-frequency ones. As a forward-traveling wave approaches a region most sensitive to its frequency, the wave slows, grows in amplitude, and stimulates hair cells sitting atop the membrane. Those cells then move in concert and change shape—much like piezoelectric devices when a voltage is applied across them—and coherently amplify that frequency component of the signal. A separate group of hair cells then chemically relays the amplified signal to the auditory nerve.
The foundation for that picture—the notion that the cochlear partition acts as a transmission line carrying energy as a traveling wave along the spiral—was established as early as the 1920s. And Georg von Békésy’s experiments on waves in the cochleae of animals and human cadavers in the 1930s and 1940s later won him the Nobel Prize in Physiology or Medicine and set the stage for subsequent cochlear models.
Recent work performed by Tianying Ren and colleagues at the Oregon Health and Science University, Xi’an Jiaotong University in China, Karolinska Institute in Sweden, and the University of Michigan may prompt theorists to rethink at least part of the cochlear mechanics developed in the past 70 years. Using interferometry to measure the dynamics of the basilar membrane in live animals, they offer evidence that energy travels directly through the cochlea’s fluids as well as along its membrane. The evidence came from addressing a fundamental question: How do waves propagate backward in the ear? 5
Otoacoustics
In 1978 biophysicist David Kemp made a remarkable discovery. 2 The cochleae in live animals not only process external sounds but also create their own, which leak back out through the stapes and eardrum. Those otoacoustic emissions are a normal byproduct of the ear’s amplification process and distinguish a healthy cochlea from an impaired one. Detecting OAEs in the lab is as simple as listening for spontaneous tones, echoes, or distortions that emerge from the ear, sometimes in response to certain frequencies. Distortion, for example, refers to the nonlinear response of the cochlea to the mixing of different-frequency sounds introduced to the ear. Today clinical OAE measurements are a routine part of audiometric tests, and our ability to disentangle the emitted frequency components makes them powerful diagnostics. Indeed, nearly every infant born in the US is tested for OAEs.
Partly because the emissions involve rather long time delays, researchers, including Kemp, assumed that sound would just travel backward along the membrane in direct analogy to a normal forward-traveling wave. In 2004 Ren tested the assumption on young, anesthetized Mongolian gerbils. 3 After implanting a microphone and earphone in the ear canal, he surgically exposed each gerbil’s cochlear partition to light from a laser interferometer. The partition’s sound-induced vibration was detected from the Doppler shift of the reflected light. With the interferometer’s voltage output proportional to the velocity of the transverse vibration of the membrane, Ren could measure the magnitude and phase of the vibration as the laser spot was swept along the length of the membrane. That allowed him to discern the forward-traveling waves from reverse-going ones. To his surprise, Ren never observed a reverse-traveling wave.
The experimental approach provided information on membrane vibrations at many locations along the cochlear spiral. Unfortunately, the membrane’s extremely low reflectivity, roughly 0.03%, made the interferometry data noisy. To combat the problem and ensure waveforms pronounced enough to be discernable, Ren had to use sound pressures at the level of normal conversation—some 60 to 70 dB. At those pressures, other researchers pointed out, a large forward wave would always mask the presence of a reverse wave on the membrane. Another problem had to do with the proximity of the measurements to the site where the otoacoustic distortion originated. If the two locations on the membrane were not separated enough, a mixture of forward- and reverse-traveling waves produced in the same vicinity would, the argument went, also obscure the results.
Compression by default
In a newly updated experiment, Ren and company removed part of the bone surrounding the cochlea and implanted gold-coated glass beads in two spots about 200 microns apart along the cochlear partition. 1 With more than a hundredfold improvement in reflectivity, the interferometry could detect vibrations less than a picometer in amplitude and allowed the team to use sound pressures on the scale of a whisper.
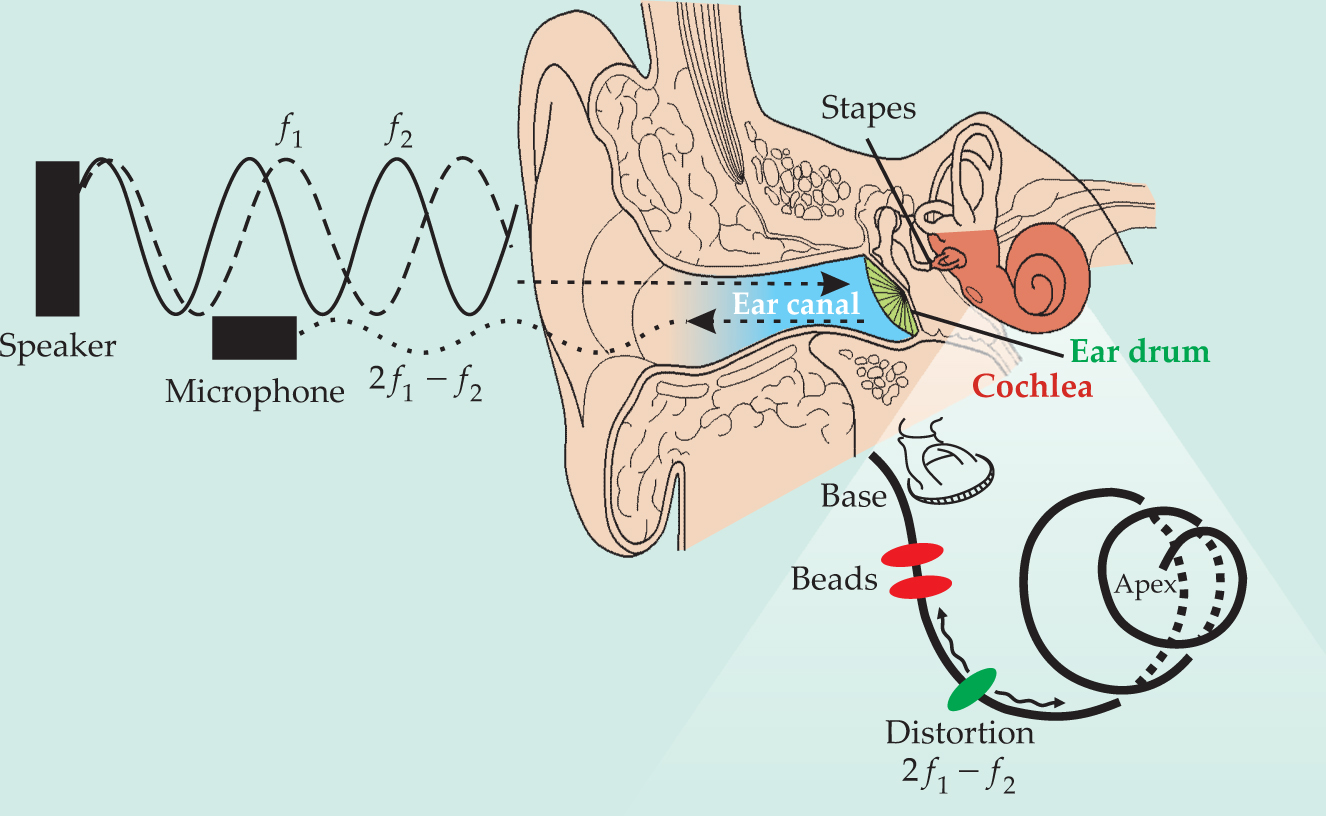
To create reverse-traveling waves, the team introduced two tones at frequencies f 1 and f 2 into the ear with values designed to generate OAE distortion at a frequency well downstream of the bead locations. Interferometry should then pick up a ripple in the membrane as the wave travels backward (see the figure). Using a series of frequencies (with f 2/f 1 fixed), they repeatedly measured the membrane’s responses at the two beads and then Fourier-transformed them to obtain the vibrational magnitude and phase over a wide range of cochlea-generated frequencies 2f 1 − f 2.
The results confirmed the 2004 finding. In each measurement, the researchers found only what looked like forward-traveling waves; that is, based on the phase difference at the two spots, a wave at frequency 2f 1 − f 2 always reached the more basal location before the more apical one. In the absence of a measurable backward-going transverse wave along the membrane, Ren infers, the fluid must contain a longitudinal compression wave, moving at the speed of sound, that transports the otoacoustic energy back to the base. Because of the high speed, the reverse propagation would not introduce a significant phase lag between the two bead sites, he says. At the middle ear, the fast wave would be partially transmitted to the ear canal where it can be recorded and partially converted into a slow forward-going transverse wave that interferometry measures as a phase gradient at the distortion frequency.
The in–out motion of the stapes is known to trigger a compression wave in the forward direction. But how might compression waves arise downstream in the cochlear spiral? Although the cochlea certainly works as an amplifier, it’s still unclear just how the hair cells do the job or generate reverse waves, says Harvard University’s Christopher Shera. In 1980 Patrick Wilson suggested that the hair cells might behave as radiating dipoles if they could change in volume. A hair cell can certainly change shape at acoustic frequencies. But because osmotic processes are too slow to explain how a cell might swell and shrink that quickly, researchers have never taken the suggestion seriously. Shera wonders if the reverse compression-wave hypothesis might put Wilson’s idea back into play.
Even experimentally, however, the question of whether the backward wave is transverse or longitudinal remains unresolved. Columbia University’s Wei Dong and Elizabeth Olson have just published a set of in vivo measurements of cochlear pressure itself—in particular its spatial variation in the transverse direction. 4 Intriguingly, the distortion pressure decreased with distance from the cochlear partition, a result that favors a slow reverse-wave interpretation. Were the space-filling compression pressure dominant, one should expect to observe it. “It may be there, but if so, it’s within our noise,” says Olson.
Ren summarizes the current state of the field succinctly: “Very exciting, yet very frustrating.”
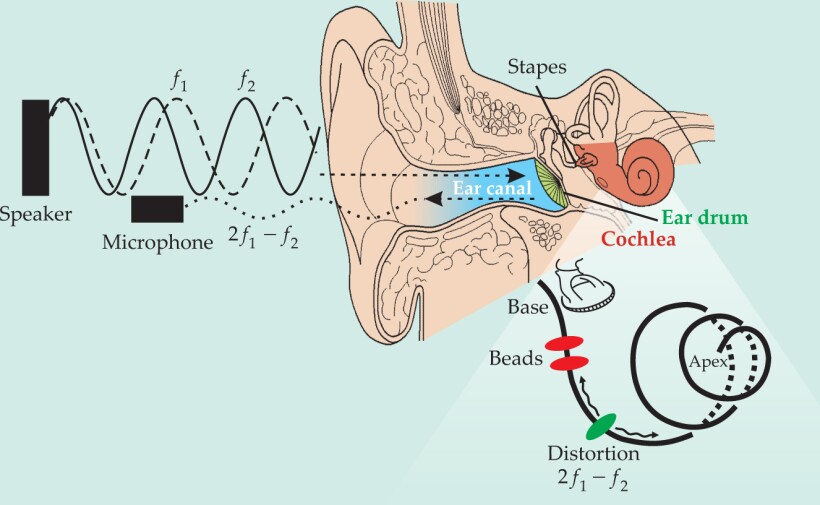
Driving sound backward in the ear. The nonlinear behavior of the inner ear gives rise to sound waves that emerge from the cochlea and travel outward into the ear canal. Are the sound waves slow, transverse waves that ripple along the basilar membrane (black spiral), or fast, compressive ones in the fluid? To find out, Tianying Ren and colleagues surgically implanted reflective gold beads at two spots (red) along the membrane of a gerbil and used laser interferometry to measure the magnitude and phase of the membrane’s vibration there. To drive the cochlea backward, the team sent in two primary tones at frequencies f 1 and f 2 low enough that the nonlinear distortion frequency 2f 1−f 2 produced by the cochlea should arise downstream (green) of the interferometry measurements. Significantly, they never observed a reverse transverse wave.
(Adapted from refs. 3 and 5.)
References
1. W. He, A. Fridberger, E. Porsov, K. Grosh, T. Ren, Proc. Natl. Acad. Sci. USA 105, 2729 (2008). https://doi.org/10.1073/pnas.0708103105
2. D. T. Kemp, J. Acoust. Soc. Am. 64, 1386 (1978). https://doi.org/10.1121/1.382104
3. T. Ren, Nat. Neurosci. 7, 333 (2004). https://doi.org/10.1038/nn1216
4. W. Dong, E. S. Olson, J. Acoust. Soc. Am. 123, 222 (2008). https://doi.org/10.1121/1.2816566
5. T. Ren et al., J. Neurophysiol. 96, 2785 (2006). https://doi.org/10.1152/jn.00374.2006




