Imaging a mouse’s brain through its skull
DOI: 10.1063/PT.3.2900
Biological tissues are rarely transparent. That fact has long complicated efforts by optical microscopists to resolve deeply embedded features, because the light waves used for imaging are scattered throughout the intervening tissue. The deeper the object, the more blurred the image. To ameliorate the problem, scientists in recent years have turned to adaptive optics: Using rapid, real-time analysis of a distorted-light signal, a computer-controlled deformable mirror or spatial light modulator compensates for aberrations in the optical path by reshaping the waves and thereby restoring crisp image detail.
Conventionally, the deformable mirror is placed at the microscope’s so-called pupil plane—the back focal plane of the objective lens—where the mirror applies a uniform correction everywhere in the image plane. Such placement is appropriate, and provides the best focus, when the aberrations are spatially invariant, as is the case, for instance, when a refractive-index mismatch occurs along a flat interface. However, that condition doesn’t hold in turbid biological tissue; and unless the deformable mirror actively adjusts the wavefront for different scanning angles through the tissue, a position mismatch develops between distortion and compensation. The result is a good focus over a very small field of view.
Like astronomers before them, microscopists can benefit from virtually placing the wavefront modulator in the scattering layer itself, where a point-to-point correspondence exists between mirror deformations and the aberrations they correct. 1 Meng Cui and collaborators at the Janelia Research Campus of the Howard Hughes Medical Institute have now applied that scheme, known as conjugate adaptive optics, to brain imaging. By optically projecting a deformable mirror’s corrective wavefront onto the bony outer surface of a mouse’s 150-µm-thick skull, they obtained diffraction-limited images of neurons and microglia—a type of brain cell with thread-like extensions—another 50 µm under the skull, 2 as shown in the figure.

A restrained mouse, awaiting anesthesia and highly focused near-IR light pulses to its brain, sits under the objective lens of a microscope. The genetically engineered mouse has green fluorescent protein in its microglia—brain cells responsible for the animal’s immune response to injury and to pathologies such as Alzheimer’s disease and tumors; the cells fluoresce in response to intense near-IR light excitation. Collecting the fluorescence in a photodetector (not shown) from a scan of the focus through an 8000-µm3 volume of the brain maps out a three-dimensional image. Thanks to a new adaptive-optics scheme, fine details such as the threadlike branches of a single microglial cell are resolved (see inset’s 2D projection). (Photograph courtesy of Meng Cui; inset adapted from ref.
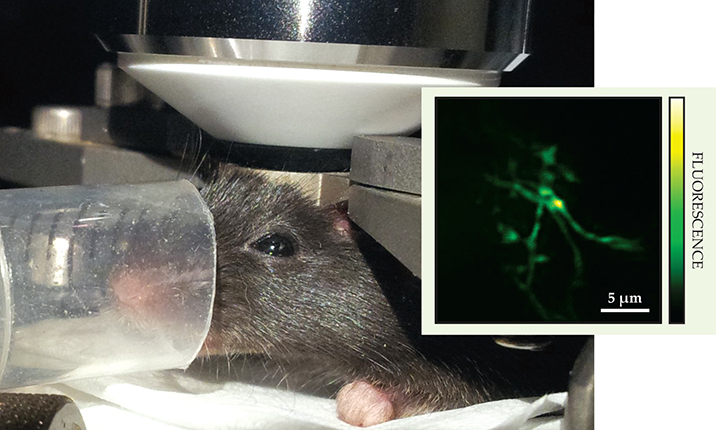
To resolve those cells previously, researchers had to thin the skull or remove it entirely. But noninvasive imaging is strongly preferred because microglia cells serve as the primary immune response to any kind of brain damage or infection. The Janelia group is the first to capture their dynamics through an intact skull. What’s more, the unconventional placement of the deformable mirror bought them a 15-fold increase in corrected field of view—from an area of 20 µm2 to about 300 µm2.
Coming into focus
The achievements build on work Cui began three years ago to combine multiphoton fluorescence microscopy, the gold standard for in vivo deep-tissue imaging, with well-established wavefront engineering. 3 In two-photon fluorescence microscopy, the wavelength of laser light used to excite target molecules is in the near-IR, long enough that it suffers far less scattering than visible light and isn’t strongly absorbed by hemoglobin in the blood. And because the excitation probability is extremely low, the only molecules that fluoresce are those in the tightly focused volume of tissue where the IR light intensity is highest. The two-photon technique thus reduces out-of-focus excitations that would otherwise hinder wide-field imaging.
In their new work, Cui and his colleagues installed a photodetector to pick up the fluorescence signal as it leaked out of the brain and back through the objective lens. They then iteratively changed the wavefronts of the illuminating near-IR pulses while continuously monitoring the fluorescence intensity of the signal until they found a wavefront shape that produced the strongest signal outside the brain and thus the best focus inside it. For that iterative trial-and-error process, the team divided the deformable mirror, with its 1024 independent spatial elements, or pixels, into two halves: One-half was kept fixed to provide a reference, and the other half was repeatedly changed. The procedure turns out to be remarkably fast: The total optimization time could be reduced to less than 1.5 seconds.
Allard Mosk and Ivo Vellekoop from the University of Twente in the Netherlands developed an earlier version of the waveshaping technique in 2007. They focused a portion of light through a layer of white paint onto a photodetector and realized a thousandfold increase in transmission intensity after optimizing the light’s wavefront (see reference and Physics Today, September 2008, page 20
Fortuitously, Cui and his collaborators only had to optimize their deformable mirror occasionally. Because the skull is so stable an environment, they were able to scan the brain for hours—with each three-dimensional scan requiring just 21 seconds—to capture some cellular dynamics before reoptimizing the shape of the incident near-IR waves.
“This is a step forward, but not the end,” Cui concedes about his demonstration. “We would like a still larger field of view—at least 10 000 µm2.” Ordinary adaptive optics, whether conventional or conjugate, is most effective when a single, thin aberration layer is dominant, and it breaks down when there are multiple layers. The skull has denser bone near its inner and outer surfaces than in its center, which complicates proper waveshaping. “Incorporating multiple deformable mirrors, a technique now common in astronomy, into a microscope system to account for multiple layers would be operationally extremely difficult,” says Oxford University’s Martin Booth. “But I’m sure someone will do it eventually.”
References
1. J. Mertz, H. Paudel, T. G. Bifano, Appl. Opt. 54, 3498 (2015). https://doi.org/10.1364/AO.54.003498
2. J.-H. Park, W. Sun, M. Cui, Proc. Natl. Acad. Sci. USA 112, 9236 (2015). https://doi.org/10.1073/pnas.1505939112
3. See, for example, J. Tang, R. N. Germain, M. Cui, Proc. Natl. Acad. Sci. USA 109, 8434 (2012). https://doi.org/10.1073/pnas.1119590109
4. I. M. Vellekoop, A. P. Mosk, Opt. Lett. 32, 2309 (2007).https://doi.org/10.1364/OL.32.002309




