Cold, supersaturated urban air could be accelerating pollutant particle growth
DOI: 10.1063/PT.3.4517
In early April, city dwellers of India’s northern provinces experienced once-in-a-lifetime views of the snow-capped Himalayas, thanks to a rare absence of smog. The foggy mixture of particles and gases will certainly reappear in the region once coronavirus restrictions are lifted and the air pollution returns. Particulate matter in the atmosphere is a leading cause of lung disease and may contribute to neurological diseases such as Alzheimer’s. A better understanding of the pollutants that lead to smog formation is necessary for any mitigation effort to succeed.
Some particles in the atmosphere, called primary particles, form directly from combustion or mechanical generation. Other particles, called secondary particles, form from trace gases that condense and stick to the surfaces of existing particles, and sometimes undergo complex chemical evolution. Winter smog, shown in figure
Figure 1.

Smog looms over Prague on a February day. The pollutant, which forms when particulate matter reacts with UV radiation in the atmosphere, is a major contributor to respiratory disease. Still, it’s unclear how the large particles found in smog can grow in urban air. (Courtesy of Vojife/Wikimedia Commons, CC BY 3.0

Now Neil Donahue of Carnegie Mellon University and colleagues have shown in a laboratory experiment that common nitrogen-containing vehicle emissions may accelerate the particle-formation process. 1 Those compounds have previously been thought to exist in concentrations near their equilibrium values in the atmosphere and therefore not to play a role in particle formation or early growth. The researchers identified conditions under which the vapor pressures of precursor pollutants in cold temperatures, typical in some winter months and in the upper atmosphere, allow nitric acid and ammonia to condense and quickly grow into large secondary particles of ammonium nitrate. Those particles can add significantly to the total number of particles in the atmosphere and contribute to the associated health effects.
Growing pains
Internal combustion engines, incinerators, cooking fires, and other sites of combustion in cities lead to high atmospheric concentrations of gas-phase pollutants, many of which readily condense into tiny clusters of molecules a few nanometers in size. But it’s not easy for a small cluster of molecules to grow enough to form a larger stable particle, typically characterized as a cluster 10 nm or larger.
Many nascent clusters simply evaporate due to an effect identified in 1871 by Lord Kelvin: Smaller clusters have greater curvatures than larger ones, and therefore tend to have higher surface vapor pressures. As a result, smaller clusters evaporate more readily. For molecular clusters with diameters less than about 10 nm, molecules are more likely to escape than remain stuck together.
Other newly formed clusters are scavenged by existing particles. As tiny clusters grow, they reach a size where they can get caught in the turbulent wake of a larger, existing particle and stick to its surface. As a result, the total number of particles does not increase. The scavenging of new clusters by existing particles is known as the condensation sink rate.
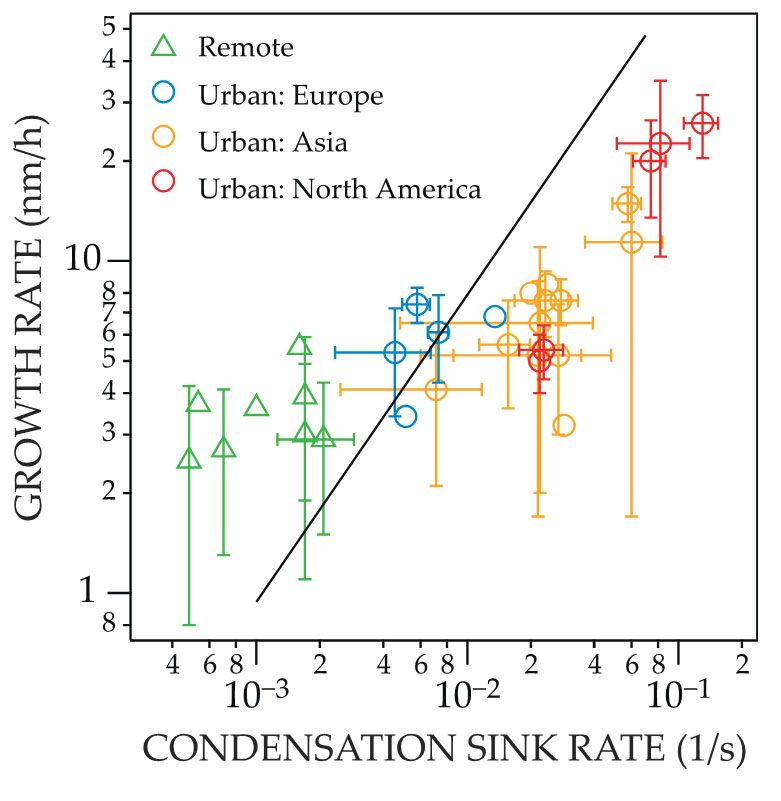
The ability of small, newly formed clusters to grow into larger, stable particles depends on the ratio of the condensation sink and the cluster growth rate. Observations from Europe, North America, and Asia, summarized in figure
Figure 2.

Atmospheric particle growth rates, shown here on the vertical axis, are only modestly faster in many urban environments (red, orange, and blue dots) than in remote ones (green triangles). The condensation sink rate (horizontal axis) describes how quickly newly formed clusters are scavenged by existing particles and is higher in urban environments than in remote ones. The persistence of those clusters depends on the ratio of the condensation sink and growth rates (the slope of the black line corresponds to a constant ratio), with small urban particles likely to be lost by scavenging unless they grow quickly. (Adapted from ref.
Cows, cars, and CLOUD
Ammonia is the most significant base gas in the atmosphere, where it contributes to the formation of ammonium nitrate in the presence of nitric acid. In rural areas, ammonia is closely associated with livestock dung; in urban areas, vehicle catalytic converters may generate a level of ammonia similar to that produced in some agricultural regions. 2 The most common pathway for the formation of ammonium nitrate in a relatively clean atmosphere keeps the compound near chemical equilibrium with gaseous ammonia and nitric acid. As a result, that mechanism is unlikely to increase the number density of particles unless the atmosphere becomes supersaturated with nitric acid and ammonia.
To investigate what conditions may drive supersaturation and new particle formation in an urban setting, Donahue and his colleagues studied mixtures of common pollutants in the CLOUD (Cosmics Leaving Outdoor Droplets) chamber at CERN (figure
Figure 3.

The CLOUD (Cosmics Leaving Outdoor Droplets) chamber at CERN is a 26-cubic-meter tank in which temperature and gas concentrations are carefully controlled to simulate natural atmospheric conditions between the ground level and the stratosphere. The proton synchrotron serves as an artificial source of cosmic rays, which simulates natural atmospheric conditions and may stabilize particles that form from the ionized gases in the chamber. External instrumentation allows for constant monitoring of the chamber’s contents. (Courtesy of CERN/Neil Donahue.)

In a series of experiments designed to replicate winter conditions in polluted cities, the researchers introduced various combinations of nitric acid, ammonia, sulfuric acid, and their precursor molecules into the CLOUD chamber. They exposed the gases to a range of temperatures typical of the lower atmosphere and varied the radiation input to control ion distribution. Mass spectrometers and particle counters that encircle the chamber determined the resulting concentration of gases and the properties of newly formed particles.
The team found that at temperatures warmer than –15 °C, ammonium nitrate and sulfuric acid followed a known pathway to form small clusters of molecules. 3 Once clusters of ammonium sulfate reached a threshold size, ammonium nitrate began to condense onto the clusters, resulting in rapid particle growth—above 100 nm/hr—as long as the temperature remained below 5 °C. At temperatures colder than –15 °C, which can occur in humid air outflows above some clouds, the nitric acid and ammonia gases nucleated through an acid–base stabilization mechanism to form particles of ammonium nitrate, which then grew via additional condensation of ammonium nitrate.
Calculations showed that the critical size at which ammonium nitrate begins rapid growth depends on the maximum concentrations of the ammonia and nitric acid. 4 Once that size is reached, rapid growth continues until the precursor vapors return to the chemical equilibrium commonly found between ammonia, nitric acid, and ammonium nitrate.
Large-scale atmospheric transport models do not take into account the newly proposed path to particle formation. The CLOUD experiments suggest that it may be important instead to consider those vapors using dynamic calculations. The researchers propose that variations in temperature and emission sources in cold urban settings provide the conditions necessary for localized supersaturation of nitric acid and ammonia. By imposing regulations to control nitrogen emissions, urban planners could reduce the concentration and growth of particles and improve air quality.
References
1. M. Wang et al., Nature 581, 184 (2020). https://doi.org/10.1038/s41586-020-2270-4
2. B. J. Finlayson-Pitts, J. N. Pitts Jr, Chemistry of the Upper and Lower Atmosphere, Academic Press (2000).
3. J. Kirkby et al., Nature 476, 429 (2011). https://doi.org/10.1038/nature10343
4. J. Kontkanen et al., Atmos. Chem. Phys. 18, 13733 (2018). https://doi.org/10.5194/acp-18-13733-2018




