Chemistry Nobelists developed reactions that are “compatible with almost everything”
DOI: 10.1063/PT.3.5134
Molecules aren’t Tinkertoys. Chemists can’t just pluck atoms out of a box and connect them however they want. Rather, they rely on an inventory of reactions, accumulated over generations of research, for manipulating molecular structures. Building a new molecule means solving an intricate puzzle of which reactions to perform in which order.
Those reactions can be temperamental. They can depend sensitively on solvents, temperatures, and other parameters. The reactants don’t always find each other, and they don’t always react as planned. In a complex environment, they often react with the wrong thing entirely to form unwanted by-products. In a multistep synthesis of a complicated molecule, the inefficiencies quickly compound, and chemists often need an enormous amount of starting material to make even a tiny amount of product.
Carolyn Bertozzi DO PHAM/STANFORD UNIVERSITY Morten Meldal LARS KRABBE K. Barry Sharpless IKE SHARPLESS





A few special reactions buck the trend. Their reactants seek out and react only with each other, with nearly 100% efficiency, regardless of what other molecules might be around. This year’s Nobel Prize in Chemistry recognizes three researchers who made key contributions to developing and harnessing the power of those ultraefficient reactions: Carolyn Bertozzi of Stanford University, Morten Meldal of the University of Copenhagen, and K. Barry Sharpless of Scripps Research.
Sharpless coined the term “click chemistry” to describe the reactions, likening the joining of molecules to the satisfying “click” of a push-buckle tab inserted into its socket. Bertozzi introduced the term “bioorthogonal chemistry” to emphasize how the reactions can be so indifferent to their surroundings that they can be carried out in living cells, or even living animals, with no ill effects.
The distinction between the two terms is largely in the application; the reaction properties they refer to are very similar. “We talk about reactions that are compatible with biology,” says UCLA’s Ellen Sletten, who earned her PhD under Bertozzi in 2011. “But really we design these reactions to be compatible with almost everything.”
Beyond its use in chemistry, biology, and related fields such as materials and polymer science, click chemistry has been a great benefit to scientists in many other disciplines, including physics, by making the tools of chemistry accessible to researchers who aren’t trained as chemists. “The main selling point is that it’s really easy to do,” says Katie Bratlie of the National Academies of Sciences, Engineering, and Medicine, “so it’s really beneficial for lots of applications.”
Seeing sneaky sugars
The story’s molecular protagonist is the azide, a group of three nitrogen atoms bound together as part of a compound or larger molecule. When made into a sodium salt, azide has been used as the active ingredient in car airbags. Sodium azide is a stable solid until it’s heated, when it rapidly decomposes to release nitrogen gas. For azides in organic molecules, the chemical details differ, but the effect is similar: The azide is nearly inert, but it carries a lot of pent-up potential energy. So when it finds the right reaction partner, it’s ready to react vigorously.
But there aren’t a lot of azide reaction partners. It’s what’s known as a soft reactant: Its charge density is spread out, and it’s highly polarizable. Chemistry—and especially biology—doesn’t have many other soft reactants; most reactants are hard, with concentrated, relatively unmovable charge distributions. Because hard reacts with hard and soft with soft, azides don’t react with much.
In the late 1990s, Bertozzi started to recognize azides’ potential. She was interested in glycans, the complex sugars that coat the outsides of cell membranes, about which little was known. To study the behavior of a new biomolecule in a cell, a good first step is typically to label the molecule with a fluorescent tag and then directly observe where it is and what it’s doing. For proteins, the labeling could be done through genetic engineering (see Physics Today, December 2008, page 20
The key is that cells are lazy in constructing their glycans. They don’t build the complex sugars atom by atom. Rather, they take the simple sugars that they ingest, such as glucose and galactose, and assemble the glycans directly from those building blocks. Bertozzi discovered that if she fed a cell a sugar molecule bound to a chemical group that’s not supposed to be there, the cell still inserted the sugar, unchanged, into one of its glycans—as long as the unnatural group was sufficiently small and unreactive. Azides fit the bill, and she started tricking the cells into making their own azide-tagged glycans.
Azides by themselves aren’t fluorescent. To complete the fluorescence labeling, Bertozzi needed to flood the cell with a fluorescent dye bound to something that reacts with the azide. In those early years, she used a reaction called the Staudinger ligation, in which the azide reacts with a phosphorus atom bound to a benzene ring. 1 But although the Staudinger ligation was bioorthogonal—it could harmlessly label glycans in cells and even in live mice—it wasn’t especially fast: Labeling a glycan took the better part of a day, and the benzene–phosphorus molecule was degrading and getting eaten by cells almost as fast as it was reacting with the azides. Clearly, a different azide reaction partner would be better, if one could be found.
Function over structure
Meanwhile, Sharpless was formulating a bold vision of what he hoped would be a new way for chemists to think about organic synthesis. In the conventional approach, chemists begin with the end in mind: If they want to create, say, a new pharmaceutical, they first identify the precise molecular structure they want to make, and then they figure out how to make it. The drawback of that strategy is that even if they succeed in making the target molecule—which may take years—the synthesis could be highly inefficient. If the ultimate goal is to manufacture the new substance on a commercial scale, they may be dooming themselves to an extremely expensive end product.
Sharpless’s idea, which he articulated in the 2001 paper that introduced the term “click chemistry,” was to change the focus from structure to function. 2 The space of possible molecular structures is indescribably vast; it stands to reason that for any desired function, there ought to be many different molecules that are fit for the job. Moreover, those molecules probably aren’t all equally difficult to make. Sharpless argued that chemists stood a greater chance of discovering valuable new substances by focusing on the molecules that are easiest to synthesize and taking the functions as they come.
That’s where the click reactions came in. Molecules are easy to synthesize if they can be assembled through reactions that are efficient and simple to carry out. For a reaction to retain its efficiency in the context of many different molecular assemblies, it needs to be highly specific: Its reactants should react only with each other, not with anything else that might be present.
Sharpless went on to list several candidate click reactions, although most of them fell short of the ideal of perfect efficiency and selectivity. “But the paper challenged chemists around the world to look for even more efficient reactions,” says Jeremiah Johnson of MIT. “And seeding that idea has led to transformative advances.”
Copper-catalyzed click
The quintessential click reaction, shown in figure
Figure 1.

Click reactions efficiently connect almost anything to anything else: The orange and green blobs can represent molecules, solid surfaces or particles, or even living cells. (a) The classic click reaction, discovered independently by the groups of Morten Meldal and K. Barry Sharpless, joins an azide (N3) with an alkyne (a carbon–carbon triple bond), catalyzed by copper. (b) Instead of a simple alkyne, Carolyn Bertozzi used an alkyne in a strained octagonal ring. Because it requires no toxic copper catalyst, the cyclooctyne reaction can be performed in living cells. (Figure by Freddie Pagani.)
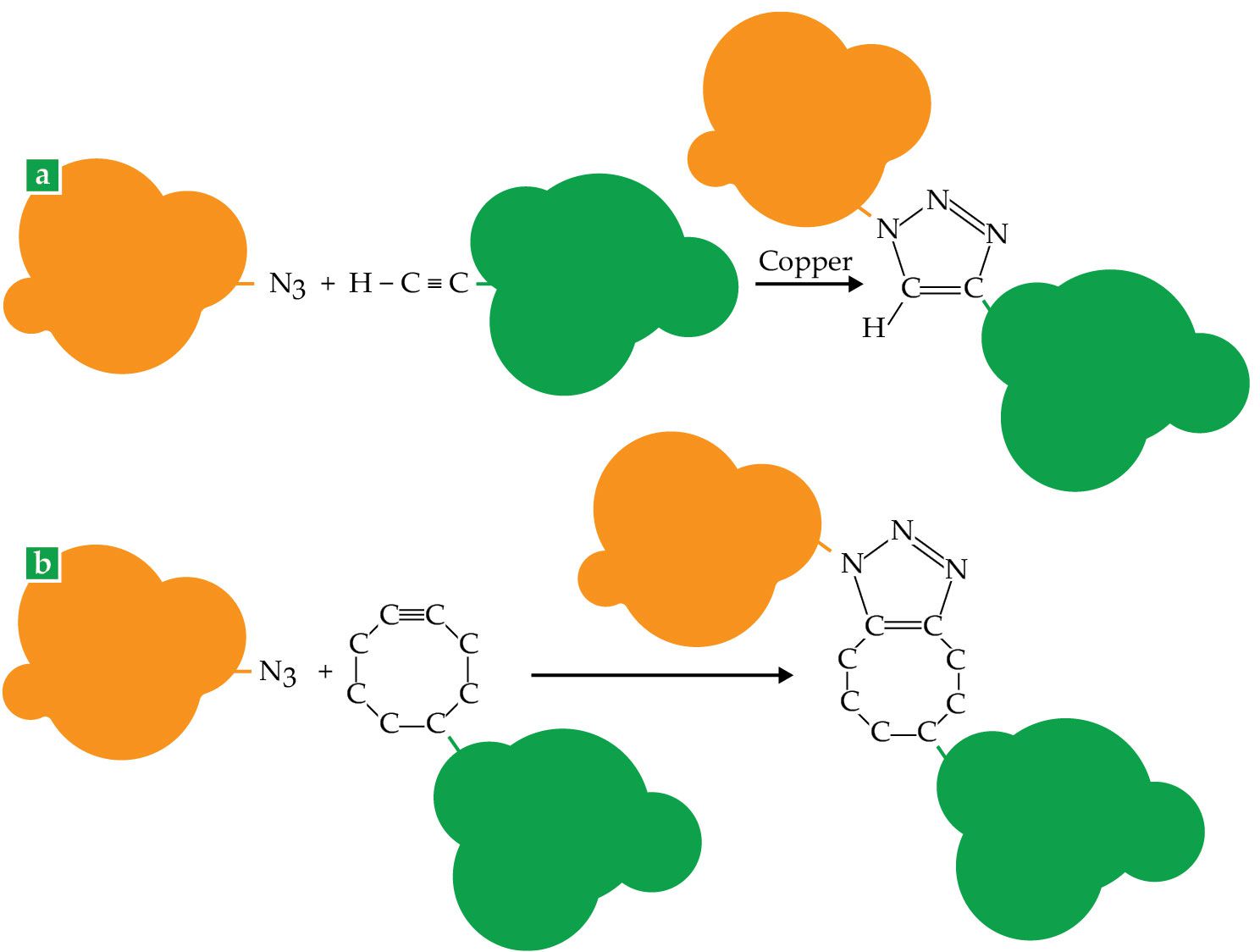
The reaction the groups discovered joins an azide with an alkyne—two carbon atoms joined by a triple bond—in the presence of a copper catalyst, to make a pentagonal carbon–nitrogen ring. The bare reaction, without the catalyst, had been well studied by generations of chemists, and Sharpless mentioned it briefly in his 2001 paper. But it was slow and required high temperatures and pressures.
The catalyst increases the reaction rate by a factor of 10 million. Although the reasons for the speedup are now understood—the electrons in the copper ions couple with uncanny precision to those of both reactants—they weren’t at the time. “We discovered the catalysis by serendipity,” says Meldal, “which I think is how most big discoveries happen. If something was easy to foresee, someone else would have foreseen it a long time ago.”
Both Sharpless and Meldal repeated the reaction with the azide and alkyne bound to many different molecules, including some that in any other context would be extremely reactive. None of them could distract the azide and alkyne from finding each other and reacting. The robust reaction could attach almost anything to anything else.
A milestone in the adoption of click chemistry came in 2004, when Craig Hawker, of IBM Almaden Research Center in California, and colleagues used the azide–alkyne reaction to synthesize a dendrimer, a tree-like branched polymer that had been extremely difficult to make. 4 “I knew click chemistry would be a big deal when the materials scientists started to use it,” says Georgia Tech’s M. G. Finn, a coauthor of Sharpless’s 2001 paper, “because they’re focused on function, and click chemistry is all about function.” Use of the reaction spread rapidly through the materials and polymer communities: for functionalizing electrodes, formulating new adhesives and self-healing materials, and more.
Among organic chemists, Sharpless’s idea of searching for function among easy-to-make molecules coexists with the traditional structure-focused philosophy of molecular discovery. Most molecules can’t be assembled purely by click chemistry, and there’s still a lot of interest in the ones that can’t. “The beauty and challenge of synthesizing complex structures remains, and it remains hugely valuable,” says Finn. “That hasn’t gone away, and it shouldn’t go away.”
Bioorthogonal explosion
Bertozzi, who was still on the lookout for an azide reaction that could improve on the Staudinger ligation, was also thinking about ways to speed up the azide–alkyne kinetics. “The copper-catalyzed reaction was useful for a lot of things, but it wasn’t useful for us,” she says, because the copper catalyst was toxic to living cells. Independently of Sharpless and Meldal, she came up with a different solution.
Digging into the literature, she found a 1961 paper published in German that described a version of the reaction shown in figure
It was no mystery why azides would react more explosively with cyclooctynes than with simple alkynes. An alkyne’s preferred structure is linear: The two triply bonded carbon atoms and the two atoms on either side of them all lie in a straight line. When that linear structure is forcibly bent into half an octagon, it endows the molecule with additional pent-up energy that’s ready to be released in a reaction.
Even so, the reaction is explosive only when the reactants are mixed in their pure form. When diluted in a biological system, they react as slowly as in the Staudinger ligation. Fortunately, cyclooctyne offered plenty of room for improvement. By decorating the edges of the octagon with other chemical groups, Bertozzi managed to speed up the reaction by a few orders of magnitude—enough to fluorescently label an azide-tagged glycan in a minute or two.
6
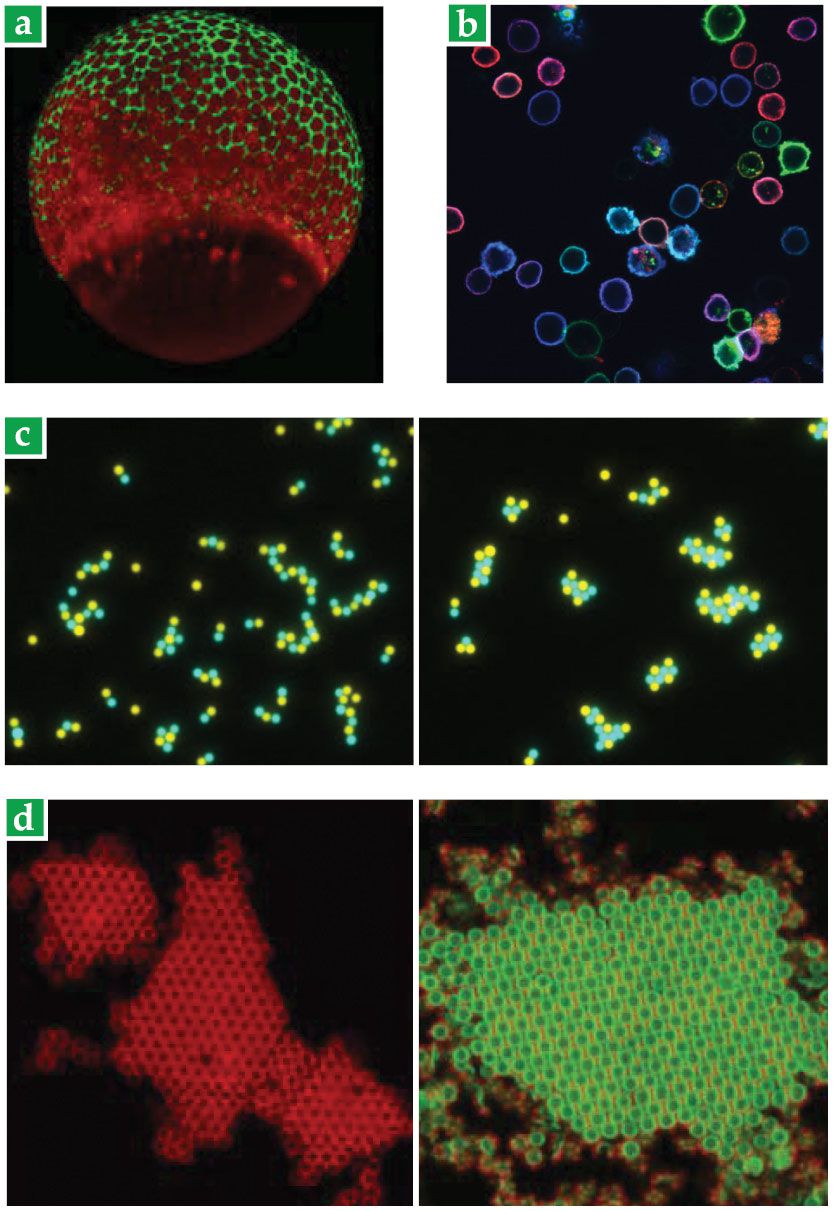
With no toxic copper required, the reaction could be performed harmlessly in living animals, such as the zebrafish embryo in figure
Figure 2.

Biology and physics applications of click chemistry. (a) Glycans in a living zebrafish embryo are tagged with a green fluorophore. (Adapted from J. M. Baskin et al., Proc. Natl. Acad. Sci. USA 107, 10360, 2010, doi:10.1073/pnas.0912081107
Finally equipped with the chemical tools to image glycans in vivo and in real time, Bertozzi and her group have gone on to gain extraordinary insights into the formerly elusive biomolecules, including their roles in animal development, immune activity, cancer, and other diseases. To translate her research into useful technologies and treatments, she’s launched nine startup companies, including OliLux Biosciences, which she cofounded with her former student Mireille Kamariza. OliLux is working to develop a test for tuberculosis—a leading cause of death in Kamariza’s home nation of Burundi—based on a molecule that’s part sugar, part fluorescent dye. The tuberculosis bacteria recognize the sugar and eat it, and the dye’s fluorescence changes once it’s in the low-dielectric-constant environment of the cell. “Unlike other tests, this detects only living bacteria,” says Bertozzi, “so you can tell if the drugs you’re using are working.”
Chemistry for all
In the 20 years since Sharpless and Meldal discovered the azide–alkyne click reaction, more reactions have joined the click-chemistry portfolio. “But they’re mostly not as robust,” says Wolfgang Binder of Martin Luther University of Halle-Wittenberg in Germany. “So when you look at the literature, there are orders of magnitude more citations for the azide–alkyne reaction than for all of the others combined.” The lone exception is the reaction between tetrazine and trans-cyclooctene, which does rival the azide–alkyne reaction in speed and selectivity; it’s been used in many recent fluorescence-labeling studies, including the one shown in figure
Vibrant research continues into many variations on the click-chemistry theme, including photoclick chemistry, in which click reactions are triggered by light; fluorogenic click chemistry, in which the reactants are not fluorescent but the product is; and a concept Johnson calls “clip chemistry,” which seeks to sever chemical bonds with the same specificity and efficiency as click chemistry forms them. 7
And the range of click chemistry’s potential uses is near limitless, because its pool of potential users is near limitless. “The reactions are very easy, even for physicists like me,” says Susumu Takahashi of the University of Southern California. Takahashi uses click chemistry to tether biomolecules to diamond surfaces so he can probe the molecules with nitrogen–vacancy centers embedded in the diamond. “Many physicists worry about working with wet labs and chemicals. Click chemistry makes everything much more accessible—and the reactions are really fun!”
“Before click chemistry, it was a nightmare,” says Jasna Brujic, a soft-matter physicist at New York University (NYU). She and her group program liquid droplets to self-assemble into larger structures, such as those shown in figure
Beyond click chemistry’s efficiency and ease of use, another benefit is that its reactants are small, explains David Pine, also at NYU. He studies the crystallization of DNA-functionalized colloidal particles, shown in figure
“Maybe click chemistry will help break down the barriers between chemistry and everything else,” says Bertozzi. “It’s really democratized chemistry.” Meldal agrees: “A very good principle is to keep it simple,” he says, “to make your work useful to a lot of people. If it’s too complicated, then nobody’s going to pay attention.”
References
1. E. Saxon, C. R. Bertozzi, Science 287, 2007 (2000). https://doi.org/10.1126/science.287.5460.2007
2. H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 40, 2004 (2001). https://doi.org/10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
3. V. V. Rostovtsev et al., Angew. Chem. Int. Ed. 41, 2596 (2002); https://doi.org/10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
C. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 67, 3057 (2002). https://doi.org/10.1021/jo011148j4. P. Wu et al., Angew. Chem. Int. Ed. 43, 3928 (2004). https://doi.org/10.1002/anie.200454078
5. G. Wittig, A. Krebs, Chem. Ber. 94, 3260 (1961). https://doi.org/10.1002/cber.19610941213
6. E. M. Sletten, C. R. Bertozzi, Acc. Chem. Res. 44, 666 (2011). https://doi.org/10.1021/ar200148z
7. P. Shieh et al., Chem. Rev. 121, 7059 (2021). https://doi.org/10.1021/acs.chemrev.0c01282
More about the authors
Johanna L. Miller, jmiller@aip.org




