Carbon capture may be a ways off, but ARPA–E is working on it
DOI: 10.1063/PT.3.1974
Engineers at aerospace company Alliant Techsystems (ATK) saw an opportunity in a condensation problem they were having while testing components in a supersonic wind tunnel: They realized that carbon dioxide in combustion gases would freeze if it was compressed sufficiently and accelerated to three times the speed of sound. The company is now wrapping up a three-year project funded with a $2.7 million grant from the Department of Energy’s Advanced Research Projects Agency–Energy. A supersonic inertial CO2 extraction system from ATK is one of 15 technologies that have been advanced by ARPA–E for their potential to remove CO2 from the exhaust of coal-fired power plants.
The goal of ARPA–E’s $40 million, three-year Innovative Materials and Processes for Advanced Carbon Capture Technologies (IMPACCT) program is to dramatically reduce the cost of extracting CO2 from flue gases so it can be sequestered from the atmosphere. All carbon capture technologies consume energy; the current benchmark technology, an absorber–desorber process that uses a monoethanol amine (MEA) solvent, costs around $90 per ton of CO2 captured—which would add as much as 50% to the cost of producing electricity from coal.
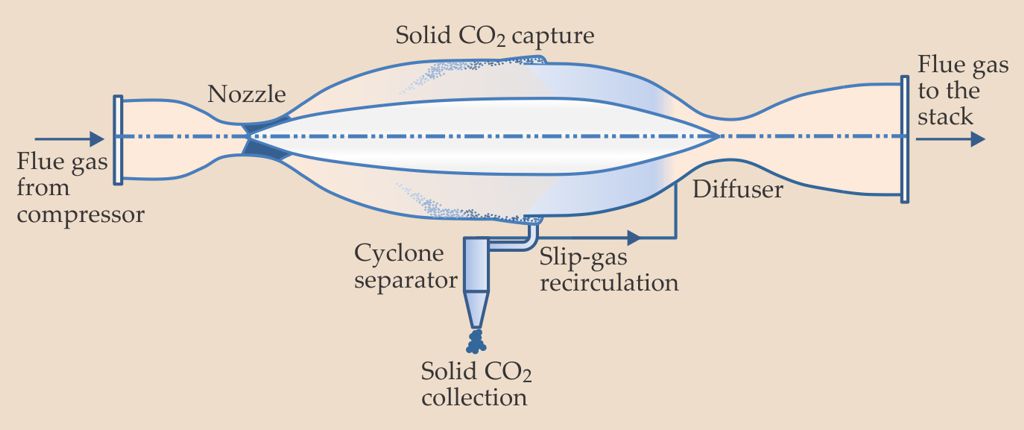
In ATK’s system, flue gas is compressed and then accelerated through a special nozzle to supersonic velocities (see figure on page 24). The process produces a rapid decrease in temperature and pressure and a deposition of CO2. The device proposed by ATK is mechanically simple, contains no moving parts, and is readily scalable. The company projects low capital costs for system construction, and operating costs are estimated to be significantly less than membrane- and absorption-based alternatives.

A carbon capture process under development at Alliant Techsystems accelerates compressed flue gas through a nozzle to supersonic speeds. The CO2 desublimates and is collected as dry ice. The technology is one of 15 novel approaches to carbon capture that have been supported by the Advanced Research Projects Agency–Energy.
ATK
According to ATK claims, the company’s process would cost $48 per ton of CO2. Other grantees claim similar costs. But Karma Sawyer, IMPACCT program manager, cautions that extrapolating cost-per-ton figures from bench-scale demonstrations—the current status of most CO2 capture projects—“is not a trivial calculation to make.” Her program adopted a goal set by DOE’s Office of Fossil Energy in 2008: to limit the cost increase for electricity generated with coal to 35%. “I can say that our technologies have compelling arguments to make” on reducing the costs, she says.
Price tag required
Sawyer says IMPACCT’s objective is “to bring new approaches, both from a materials perspective and from a process perspective, to what already was an extremely difficult goal that was set by Fossil Energy. It was very much aimed toward building a community of scientists that was not familiar with this particular applied R&D problem.”
No matter how much more efficient it can be made, CO2 capture will always add to the cost of making electricity. That means it won’t be commercially viable—beyond some limited markets such as enhanced oil recovery—until a price is imposed on CO2 emissions. Roger Aines, principal investigator on a Lawrence Livermore National Laboratory CO2 capture project funded by ARPA–E, says he’s not expecting development to be fast. “It’s time to develop the basics and get things right,” he says, noting that capture technology was even less far along when the Obama administration pushed for a cap-and-trade system in 2009. Aines’s process uses microcapsules composed of an elastomer that is permeable to CO2. Inside the 400- to 500-micron-diameter capsule are a liquid carbonate and a synthetic catalyst acting in the same way as the human carbonic anhydrase enzyme that helps separate CO2 from blood and tissue in the breathing process. The catalyst speeds the carbonate’s capture of CO2. Heating the capsules in the presence of nitrogen drives off the CO2, which can then be compressed for storage.
Another IMPACCT awardee, Sustainable Energy Solutions, would use cryogenics to condense CO2 from compressed flue gas. The gas is cooled in heat exchangers, including a proprietary desublimating heat exchanger that freezes and separates most of the CO2 from the gas stream. The work of compression is partially recovered as the gas expands through a turbine, and additional CO2 is captured through a second cooling and deposition step. The dry ice is then used to cool the incoming compressed gas stream. At the bench scale, it’s capturing 90% of the CO2 from simulated flue gas and uses just half the energy of the amine process, says company president Andrew Baxter. A pilot-scale facility is about four years away, he says.
Phase changing
Phase-changing compounds called aminosilicones are being used by GE Global Research and partners to replace MEA as the CO2 absorbent. GE’s Robert Perry says the aminosilicones change from liquid to solid on contact with CO2. Because the solid absorbent contains a higher percentage of CO2 than the liquid MEA, the energy efficiency of the process is improved. The project’s goals are to reduce parasitic power requirements to less than 23% and lower the cost to $60 per ton of CO2 removed.
There’s a downside to the aminosilicones approach, Perry admits: It’s more difficult to move a solid partway through the process loop compared with a liquid. On the other hand, having a stable solid that can be heated to 160 °C or so to drive off the CO2 at 5–6 atmospheres means that less compression is required to liquefy the CO2 for transport.
The University of Notre Dame also is developing phase-changing liquids. In that process, says Stephen Takach, managing director of the Center for Sustainable Energy at Notre Dame, enough water and residual CO2 are left after desorption to carry the phase-changing solid ionic salt back to the absorber in a slurry.
As with other CO2 capture projects, it isn’t clear what will happen to the Notre Dame project when the ARPA–E money runs out. “We’re in the stage of building a demo unit, and our plan is to operate it in the May timeframe and acquire data from the test run. In that process it will become clearer what the natural next steps are,” says Takach. Options include applying for a grant from DOE’s Office of Fossil Energy, which in March issued a broad solicitation for R&D projects through its National Energy Technology Laboratory, or partnering with industry, perhaps on a different application for the technology. In Notre Dame’s case, two of the researchers have formed a spinoff company to develop the ionic-liquids technology for refrigeration applications.
Ready to go
“The intention, from the time this program was created [in 2010], was that these projects would be positioned well to compete for a funding solicitation from Fossil Energy,” says ARPA–E’s Sawyer. “This technology requires government support in the absence of a carbon price.” She says papers from IMPACCT projects have been published in top chemical and chemical engineering journals and that a second spinoff has been created from a project at Texas A&M University.
A Columbia University project seeks to turn captured CO2 into a solid that can be easily and safely transported, stored aboveground, or integrated into products such as paper filler, plastic filler, and construction materials. In nature, the reaction of CO2 with various minerals over long periods of time—a process known as carbon mineralization—will yield a solid carbonate. But carbon mineralization as a CO2 capture and storage method is limited by the speeds at which the minerals can be dissolved and the CO2 can be hydrated. The Columbia team is using a process involving a combination of chemical catalysts to increase both rates. Ah-Hyung Park, the principal investigator with the Columbia group, says the next step will be teaming with RTI International to build a skid-mounted system for installation at a coal-fired plant.
If successful, the Columbia project would produce a long-term solid CO2 storage solution that would eliminate the worry of monitoring gas in underground storage. But that solution could have its limits; converting the gigatons of CO2 produced annually into solid form would create “mountains of carbonate everywhere,” cautions Perry.
“The technologies themselves will make this community better positioned for whatever the future does hold,” says Sawyer. “Whether they take some alternate path or not, I think that these technologies and the community of carbon capture will look very different than it did before ARPA–E made this investment.”
More about the authors
David Kramer, dkramer@aip.org




