Bending Nature’s Rules to Pattern Nanostructures on Sticky Surfaces
DOI: 10.1063/1.1784265
An important objective in surface science and modern technology is the development of simple recipes for fabricating nanostructures such as quantum dots, wires, and thin films. What makes the project challenging is that Nature places strict constraints on how atoms and surfaces interact. In general, you want the things you build on surfaces to stick. But you also want to move those same things into place. The first requires strong binding; the second, weak binding.
In 1998, John Weaver (University of Illinois, Urbana-Champaign) and collaborators resolved those conflicting requirements by developing a process that effectively replaces one surface with another. The process, buffer-layer assisted growth (BLAG), allows one to form assemblies of atoms on a weakly interacting buffer layer that can be evaporated afterward. 1 Using a buffer layer like solid xenon as a temporary proxy for the surface effectively changes the thermodynamics of the adatom-substrate system. Because atoms are weakly bound to the Xe layer through van der Waals forces, they spontaneously diffuse, bind to each other, and form three-dimensional clusters. Once formed at low temperature (20 K), the self-assembled aggregates can softly land on what may be a much more reactive surface when the buffer layer sublimates away as it warms to 80 K.
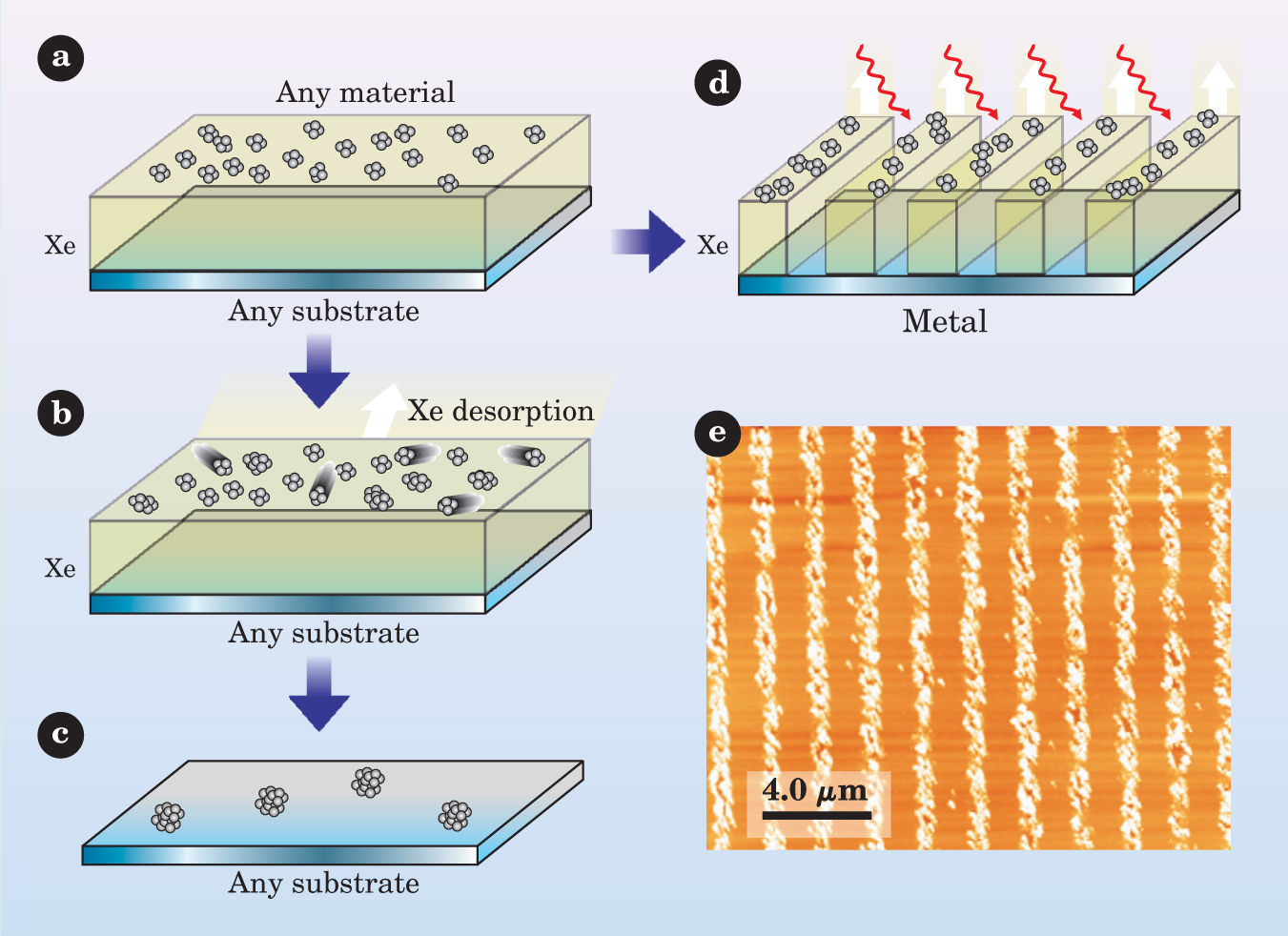
By combining this growth technique with laser-induced desorption—a process that selectively removes surface layers using the heat from a single laser pulse—Micha Asscher and his PhD student Gabriel Kerner, both from the Hebrew University of Jerusalem, have developed a new lithography method for patterning (potentially at nanometer resolution) almost anything on anything else. As proof of concept, they used the method to form submicron-wide wires of potassium on ruthenium. 2 The process, outlined in the figure, is simple.
Making gratings
The Israeli researchers had originally set out to measure the diffusivity of potassium on other metals and metal oxides as part of an investigation into catalytic processes on clean, well-defined surfaces. Nonlinear reflection and diffraction methods are especially sensitive to monolayer changes at macroscopic scales. But to follow the diffusion, they first needed to create from the deposited potassium a thin periodic grating whose spatial order relaxes under the random diffusive motion of the potassium atoms.
Kerner used the interference of a split Nd:YAG laser pulse incident on the surface to make the grating, a technique originally introduced by Ron Shen (University of California, Berkeley). The 10-ns pulse heats the metal film to selectively break the surface bonds and desorb material. The trick is to avoid damaging the well-defined Ru surface. Potassium bonds strongly to Ru, but too high a laser power would change the surface from pristine and defect-free to roughened and pockmarked with lattice vacancies, dislocations, and steps—a change that would enormously influence the diffusion of surface atoms.
Kerner and Asscher realized that using a Xe buffer layer, though, would allow them to dial down the laser power to harmless levels, leaving strips of Xe coated with K clusters that line up along the interference fringes. Ulrich Höfer and coworkers had used a complementary approach in 1997 to demonstrate that a Xe template could control the sticking of hydrogen atoms on a silicon surface. 3 For the case of metal deposition on Xe, Asscher and Kerner found that it took at least 5–10 monolayers of Xe to create a clean pattern.
The pair also realized their patterning method has applications beyond its utility for diffusion studies. When metal atoms are first deposited on the Xe buffer, they diffuse to form small clusters, comprising, perhaps, 10–20 atoms. But when the surface warms and Xe evaporates, the motion and coalescence of the newly formed clusters can be profound—in principle, even producing clusters with tens of thousands of atoms. This makes the process an effective protocol for creating clusters of controlled sizes from practically any metal. By adjusting the buffer thickness, for instance, one can potentially tailor the size and density of the nanostructures that drop to the surface, 4 as well as the profile of the grating.
Clean lithography
To measure those grating profiles directly, Asscher and Kerner switched from potassium to gold films. The first scanning force microscopy images of Au on Ru show thin wires that consist of condensed clusters (panel e). Characterizing the properties of such wires (including their conductivity as a function of wire width) remains to be done. While initially diffusing on Xe, the clusters remain balled up, essentially out of contact with the Ru. But after landing, the extent to which clusters wet the substrate depends on their relative surface energies. And the thickness and coverage of molecules could be critical to the morphology of deposited material.
By adjusting the Bragg scattering angle and the laser’s power density, Kerner has created patterns of lines that differ in widths and spacing. Because the desorption rate of ablated atoms depends exponentially on temperature, higher power densities sharpen the lines. Asscher points out that their method has the potential to form wires 30 nm wide and 5 mm in length—a 105 aspect ratio—using a single laser pulse.
In that respect, the work parallels conventional lithography: The laser wavelength accounts for how finely wires or circuit elements can be drawn. (Using an electron-beam or x-rays, even finer resolution is possible.) Columbia University’s Tony Heinz argues that what really distinguishes patterning methods like Asscher’s from more traditional photolithography is the chemical purity of the technique: “No one would claim traditional lithography is clean to the last monolayer. But this [method] is…. The xenon buffer layer vanishes without a trace.”
Asscher envisions a time when vacuum chambers might replace clean rooms in lithographic facilities. But Heinz sees the technique’s attractiveness more in terms of doing rigorous, well-controlled surface science. Fabricated in ultrahigh vacuum, for example, the patterned lines provide a template for studying carrier transport of simple molecules and exotic clusters in confined channels. But other projects come to mind as well: measuring crystal growth and diffusion of clusters of different sizes, or monitoring the reaction of one structure with another; in short, the kind of projects that prompted Asscher to develop the patterning method in the first place.

Recipe for buffer-layer growth. (a) Take any substrate and deposit a layer of cold xenon atoms on top. Follow that with atoms or molecules of your choice. Deposited on such a weak buffer layer, the atoms will interact almost entirely with just each other, as if on a skating rink. (b) Spontaneous diffusion produces three-dimensional nanoclusters. (c) Once allowed to warm, the Xe evaporates, which prompts clusters to further coalesce and softly land on the surface underneath. (d) The initial clusters can be patterned by using interference from a split laser pulse. The hot fringes ablate both the Xe buffer layer and metal clusters on top. Slow evaporation of the remaining Xe delivers the patterned clusters to the substrate, where they stick. (e) Experimental scanning force microscopy image of a gold grating (yellow) on ruthenium (orange), produced using this method.
(Panels a-d adapted from ref. 5; panel e courtesy of Micha Asscher.)
References
1. L. Huang, S. S. Chey, J. J. Weaver, Phys. Rev. Lett. 80, 4095 (1998);https://doi.org/10.1103/PhysRevLett.80.4095
for a review of early stages in the development, see J. J. Weaver, G. G. Waddill, Science 251, 1444 (1991).https://doi.org/10.1126/science.251.5000.14442. G. Kerner, M. Asscher, Surf. Sci. 557, 5 (2004).https://doi.org/10.1016/j.susc.2004.02.039
3. P. P. Williams et al., Phys. Rev. Lett. 79, 3459 (1997).https://doi.org/10.1103/PhysRevLett.79.3459
4. V. V. Antonov et al., Phys. Rev. B 68, 205418 (2003).https://doi.org/10.1103/PhysRevB.68.205418
5. J. J. Weaver, V. V. Antonov, Surf. Sci. 557, 1 (2004).https://doi.org/10.1016/j.susc.2004.03.043




