Atomic force microscopy probes fractional differences in chemical bond order
DOI: 10.1063/PT.3.1773
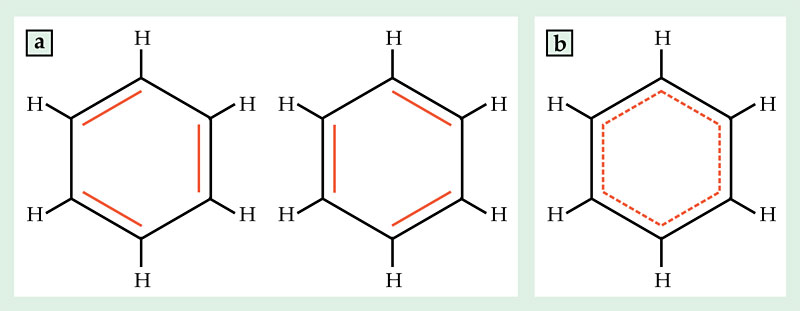
In their simplest form, covalent chemical bonds between atoms come in single, double, and triple varieties, depending on how many electron pairs the atoms share. But when electron pairs are spread among many atoms, bonds can take on fractional order, intermediate between single and double. For example, the structure of benzene can be drawn as a ring of alternating single and double bonds in two different ways, shown in figure 1a. But the actual structure is a cross between the two, shown in figure 1b, in which each bond has order 1.5.

Figure 1. Benzene is perhaps the most familiar example of fractional chemical bond order. (a) The chemical structure can be drawn in two ways as a ring of alternating single and double bonds, but (b) the actual structure is intermediate between the two, with all carbon–carbon bonds having order 1.5.
Electron pairs don’t always spread evenly among all the atoms that share them. In the soccer-ball-shaped fullerene C60, as in benzene, each carbon–carbon bond has a fractional order between 1 and 2. But unlike in benzene, not all the bonds in C60 are equivalent. Simple theory predicts that a bond shared by two of the fullerene’s hexagonal faces should have slightly higher order—0.16 more electron pairs—than a bond shared by a hexagon and a pentagon.
In the case of C60, that theoretical prediction can be verified experimentally. Higher-order bonds are shorter than lower-order bonds, and x-ray diffraction patterns of C60 crystals reveal the expected difference in length between the hexagon–hexagon and hexagon–pentagon bonds. But precision diffraction experiments are largely restricted to molecules that crystallize. For molecules that can’t be coaxed into crystalline form, or for structures that are inherently unique—such as a single defect in a graphene sheet or a molecule undergoing an unknown chemical reaction—they are of limited use.
Now Leo Gross, Gerhard Meyer, and colleagues at IBM Research in Zürich have found a way, using atomic force microscopy (AFM), to experimentally discriminate between bonds of different order. 1 They found that their technique is sensitive to bond order in two ways: through differences in electron density and, more surprisingly, through differences in bond length.
Molecules to bonds
The new results grew out of work published by the same researchers three years ago, in which they produced the first atomic-resolution AFM images of single molecules adsorbed on a surface.
2
Resolving individual atoms has been a long-standing goal of surface microscopy (see the article by Franz Giessibl and Calvin Quate in Physics Today, December 2006, page 44
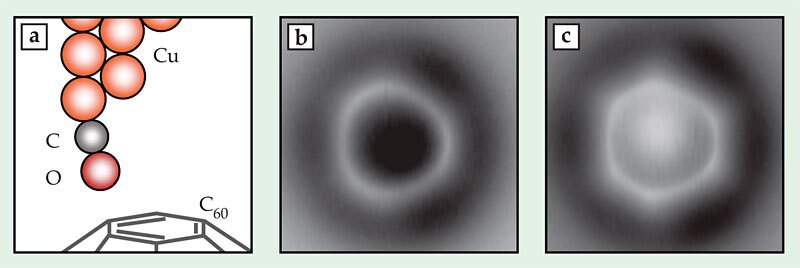
Figure 2. Imaging bonds in a C60 fullerene. (a) A copper microscope tip terminated with a single carbon monoxide molecule scans the fullerene’s topmost hexagonal face, which contains bonds of two different orders. (b) In an image taken at a height of 3.6 Å, the bonds separating two hexagonal faces (at 3, 7, and 11 o’clock) appear brighter than those separating a hexagonal face and a pentagonal face. (c) At a smaller tip height, 3.4 Å, the difference in brightness is less visible, but the hexagon–hexagon bonds appear shorter than the hexagon–pentagon bonds. Due to bending of the CO tip, the apparent length difference is 10 times the actual length difference. (Adapted from ref.
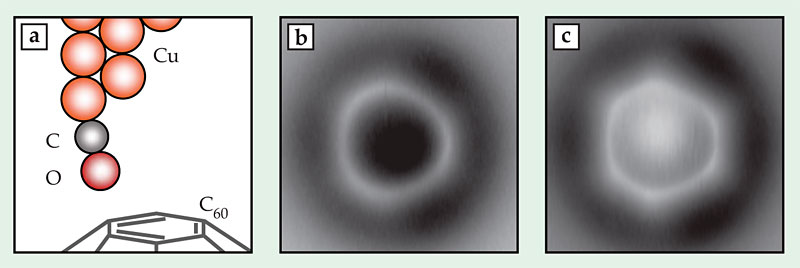
A higher-order bond contains more electrons than a lower-order bond, so a measurement that’s sensitive to electron density should also be able to measure bond order. And indeed, the IBM researchers saw some hints of sensitivity to bond order in their 2009 images, but the effect was minuscule. Three years spent refining the experiment to better tease out small differences in contrast have brought them closer to a useful technique, which they first tested on C60. Figures 2b and 2c show two images of a C60 molecule adsorbed on a surface, taken with AFM tip heights of 3.6 Å and 3.4 Å, respectively. The topmost face of the fullerene appears as a bright ring or hexagon. The researchers expected, and saw, the hexagon–hexagon bonds (at 3, 7, and 11 o’clock in the figures) to be brighter—that is, richer in electrons—than the hexagon–pentagon bonds. That difference was more apparent at greater tip heights than at smaller ones.
But at lower tip heights, the images—in which the hexagonal shape of the face was more apparent—revealed what turned out to be a lucky surprise. The higher-order hexagon–hexagon bonds are known to be shorter than the lower-order hexagon–pentagon bonds. The real difference in length—just 0.07 Å, or 5% of the bond length—would be nearly invisible in an AFM image, even with as high a resolution as the researchers achieved. But the image in figure 2c shows a distorted hexagon with edges differing in length by 0.7 Å, 10 times the real difference.
Density-functional-theory calculations performed by coauthor Nikolaj Moll showed what was going on: As the CO molecule on the AFM tip interacted with the fullerene, it bent inward, toward the center of the hexagonal face, as shown in figure 2a. The bending was stronger near the higher-order hexagon–hexagon bonds than near the hexagon–pentagon bonds. As a result, all the bonds were shifted outward in the image compared with their actual locations, and the entire hexagon appeared larger than it actually was. The hexagon–hexagon bonds were shifted by a greater distance, so (perhaps counterintuitively) they were less elongated and appeared shorter relative to the hexagon–pentagon bonds.
To test how the distortion behaved in systems more complicated than a simple hexagon, the researchers looked at two polycyclic aromatic hydrocarbons, one of which, dibenzonaphthoperylene (DBNP), is shown in figure 3a. The custom-synthesized molecules were designed to have bonds with a range of orders, and because the molecules are planar, the AFM tip could access all the bonds, not just a single face on top. The AFM images around the edges of the molecules are complicated by background forces that are hard to take into account. So the researchers focused on the interior bonds: in DBNP, the ones labeled q, r, s, t, and u.

Figure 3. Dibenzonaphthoperylene, a polycyclic aromatic hydrocarbon, shown (a) schematically and (b) imaged by atomic force microscopy. The five bonds marked q, r, s, t, and u all have different bond order, and they all appear with different brightness and apparent length in the AFM image. (Adapted from ref.
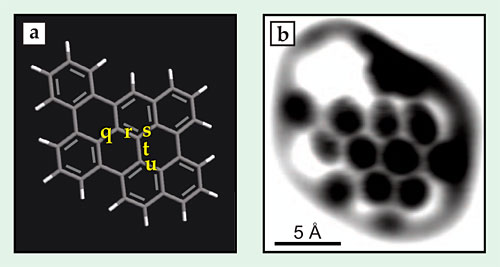
Of the five, r has by far the highest predicted bond order. It’s also both the shortest and the brightest in the AFM image in figure 3b. The other four bonds proved more difficult to distinguish: The lowest-order bond, t, appears the faintest and the second longest, but in both cases the difference is less than the experimental error.
Next steps
So far, the IBM researchers have looked only at bonds parallel to their substrate surface. That makes the researchers’ technique naturally suited to studying defects in graphene’s planar structure. Still, they’d like to extend it to nonplanar molecules; a natural next step is to look at corrugated molecules—structures that are mostly planar but have some peaks and valleys. Gross cautions, though, that the technique will always be limited to probing bonds on a molecule’s surface; bonds deep inside a three-dimensional molecule are off limits.
The researchers also hope to try using different small molecules on the end of their AFM tip. It was a fortuitous find that the CO-terminated tip can measure bond order in two independent ways; a different tip termination might yield still more complementary information.
References
1. L. Gross et al., Science 337, 1326 (2012). https://doi.org/10.1126/science.1225621
2. L. Gross et al., Science 325, 1110 (2009).https://doi.org/10.1126/science.1176210
More about the authors
Johanna L. Miller, jmiller@aip.org




