Artificial eardrums get real
DOI: 10.1063/PT.3.2836
The human eardrum is astonishingly sensitive to sound. At the threshold of hearing, it vibrates with an amplitude of mere picometers. That’s on a par with the membrane’s spontaneous oscillation from the thermal motion of air molecules bouncing off it and water molecules sloshing around inside the cochlea, the ear’s frequency analyzer and amplifier (see Physics Today, April 2008, page 26
Although typically less than 100 µm thick, the eardrum, otherwise known as a tympanic membrane, also serves as a protective barrier to the outside world. And its linkage to the chain of bones in the middle ear forms a mechanical lever that transmits sound waves from air to the liquid in the inner ear by reducing the impedance mismatch between the two media. Were it not for that mechanism, most of the energy in sound waves that enter our ears would simply be reflected.
Fortunately, most perforations of the eardrum, whether from a loud blast, say, or a rogue cotton swab, heal by themselves. But patients with chronic pathologies must endure surgeries that graft fascia (thin connective tissue), fat, or pieces of cartilage onto the eardrum—or replace it entirely—to serve as a scaffold for new tissue. The history of such intervention goes at least as far back as the 17th century, when a piece of pig bladder was the artificial eardrum of choice. And later physicians tried their luck with, among other options, vulcanized rubber, lint, tin or silver foil, or moistened cotton wool that a patient could insert daily, much like contact lenses. 1
Not surprisingly, even today’s replacements are sometimes poor substitutes. Good hearing requires a tympanic membrane that is light and elastic, yet strong enough to buffer potentially large static pressures. Our eardrums achieve those properties through a complex arrangement of collagen fibers that grow circumferentially in one of the central layers of the membrane and radially on an adjacent layer. The fibers’ near-orthogonal orientation is more likely to resist tears and lesions than an isotropic one. And the high concentration of stiff radial tissue at the drum’s center optimizes the transmission of sound waves to the middle ear’s ossicular chain.
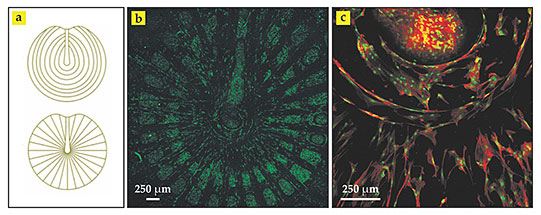
Carlos Mota (now a postdoc at Maastricht University in the Netherlands), Serena Danti (one of his PhD advisers at the University of Pisa in Italy), and their colleagues have now constructed polymer scaffolds that mimic the layered patterns and support the attachment and proliferation of living cells. 2 For the most sophisticated of their experiments, the researchers combined two technologies. Using a three-dimensional printing method known as 3D fiber deposition, they extruded a reinforcing, 300-µm-thick polymeric grid—radial or circular, as shown in panel a of the figure, or both at once—from a stainless steel syringe mounted on a movable, computer-controlled stage. They then covered the grid with a dense, 50- to 100-µm-thick mat of intersecting fibers of the same polymer via electrospinning—a century-old technology that uses an electric field to draw much finer fibers from a charged hollow needle.

Synthetic scaffolds. The collagen fibers on adjacent layers of the human eardrum follow different patterns, (a) circular and radial. Carlos Mota and colleagues superposed the two patterns to form a 300-µm-thick polymer grid and topped it with a much thinner electrospun mesh of the same material for growing biological tissue. The scaffold was seeded with human stem cells and bathed in a culture medium. (b) After eight days, it was stained and imaged using confocal microscopy. The green fluorophores reveal networks of cellular nuclei in the mesh between the grid lines. (c) This confocal microscope image of another scaffold stained with both green and red fluorophores reveals connective protein filaments known as f-actin. Near the center of the scaffold, the cell nuclei (green) and elongated protein filaments (red) appear to follow circular and radial outlines. (Adapted from ref.
Growing cells
For at least a decade, electrospinning has been one of the most popular techniques for producing thin polymeric scaffolds for tissue-engineering applications. It’s simple, is inexpensive, and produces a mesh of nonwoven, randomly oriented fibers that resemble the body’s own extracellular matrix of tissue. What’s more, the porous nature of the mesh and each fiber’s high surface-to-volume ratio provide countless sites for cell adhesion. As a transient structure, the scaffold merely maintains the spaces for target biological tissue to develop from implanted cells. The polymer degrades over a few months and is replaced by collagen.
Mota and collaborators found that scaffolds made by electrospinning alone could not control the spatial distribution of pores well enough to reproduce the structure of real eardrums. A grid, they argue, seems required to meet that challenge. Its larger, micron-scale pores act as a sieve for entrapping cells, and the additional surface area may direct cell differentiation. 3 The strategy of combining the two techniques for tissue engineering was proposed in 2008 but not implemented before now into designs for a tympanic membrane.
In one scaffold design, the researchers constructed the circumferential rings and radial spokes by combining the two patterns into a single grid layer and then covering it with electrospun mesh to form a bilayer structure. In a second, more complicated design, they constructed the patterns as two separate grids with electrospun mesh sandwiched between them—a trilayer structure. As proof of feasibility, they seeded both types of scaffold with stem cells taken from bone marrow. Within eight days of bathing the scaffolds in a culture medium, networks of cells, precursors to collagen, appeared to thrive. Panels b and c of the figure show the cells’ growth and proliferation for the simpler design, as revealed by confocal laser microscopy.
Although the trilayer design is a better simulacrum of an eardrum’s actual structure than the bilayer design, both are equally good cellular hosts when judged by the metabolic activity and the amount of DNA and protein the stem cells produced. Even so, the eight-day maturation time wasn’t long enough for collagen fibers to develop. Testing how well any eventually grown collagen fibers align with the grid lines and how well the custom-made eardrums match the acoustic properties of the real thing is on the list of next steps.
References
1. E. A. Chu, R. K. Jackler, Otol. Neurotol. 24, 507 (2003). https://doi.org/10.1097/00129492-200305000-00026
2. C. Mota et al., Biofabrication 7, 025005 (2015). https://doi.org/10.1088/1758-5090/7/2/025005
3. L. Moroni et al., Adv. Funct. Mater. 18, 53 (2008); https://doi.org/10.1002/adfm.200601158
P. D. Dalton et al., Biomater. Sci. 1, 171 (2013).https://doi.org/10.1039/C2BM00039C




