A quick and easy probe of biomolecular structure
DOI: 10.1063/pt.svur.rwqa
Structural biology has a fundamental disconnect. The biochemical world is inherently dynamic—the whole reason that proteins and other biomolecules are important is because of the functions they perform, in a complex liquid environment that’s far from equilibrium. But the main tools used to examine their structures, x-ray crystallography and cryoelectron microscopy (cryoEM), require static samples that are either crystalline or frozen.
It’s not just a conceptual separation but also a physical and logistical one. X-ray crystallography and cryoEM require different instrumentation and expertise than studies of biochemical function. Structural measurements of proteins, therefore, are performed in specialized labs by specialist researchers, often far from the chemistry and biology labs that sparked the molecules’ study.
Figure 1.

When a wandering biomolecule (purple) diffuses into a pocket trap etched into a microfluidic channel, it gets confined for a few tens of milliseconds before it can find its way back out. The average escape time, which can be accurately measured with fluorescence microscopy, depends on the molecular size and shape and on the height h1 of the exit channel. (Figure adapted from ref.
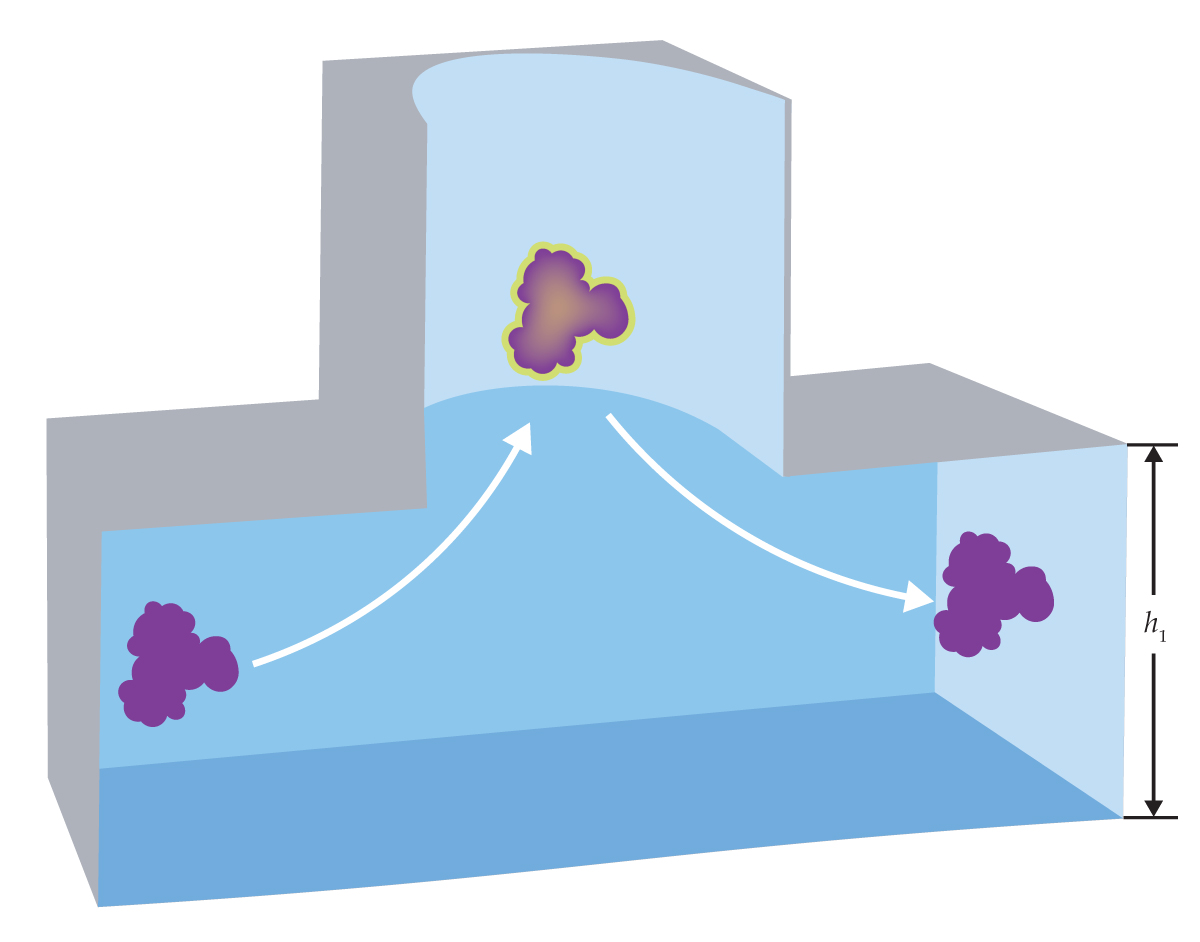
Now Madhavi Krishnan, of the University of Oxford in the UK, and her colleagues have taken a step toward bridging those gaps.
1
They’ve developed a method for gleaning some structural information about a biomolecule from the answer to a simple physical question: When a molecule diffuses into an open cylindrical pocket, like the one shown in figure
The method is by no means a replacement for x-ray crystallography or cryoEM. Measuring only a single quantity at a time yields nowhere near enough information to reconstruct a whole molecular structure. But the information it does provide is often enough to distinguish similar molecules or different conformational or chemical states of the same molecule, in some cases even if the molecular state is constantly changing.
And the technique has the considerable advantage of being able to meet biomolecules where they are: in the solution phase and in biology and chemistry labs. It requires no highly specialized equipment, and a typical measurement can be completed in one minute.
Full charge
The origin of the technique dates back to 2010, when Krishnan was a postdoc in the group of Vahid Sandoghdar, who was then at ETH Zürich in Switzerland. She and her colleagues showed that they could use cylindrical pockets for trapping nanoparticles by means of their electrical charge. 2
A technique already existed for trapping nanoparticles in solution—namely, optical tweezers (see Physics Today, December 2018, page 14
The principle is almost deceptively simple. The pockets are fabricated in a nanofluidic channel made of silica and glass. Those surfaces also pick up negative charge in solution, so there’s a repulsive electrostatic force between the particles and the channel walls. The repulsion creates a deep potential-energy well in the center of each pocket. Particles meander into the wells under ordinary diffusion, but once in, they’re prevented from escaping, and they can remain trapped for hours.
Figure 2.

Slender and chunky molecules have significantly different values of the two structural parameters that are probed with escape-time measurements: the hydrodynamic radius rH and molecular-envelope diameter Ds. (Figure adapted from ref.
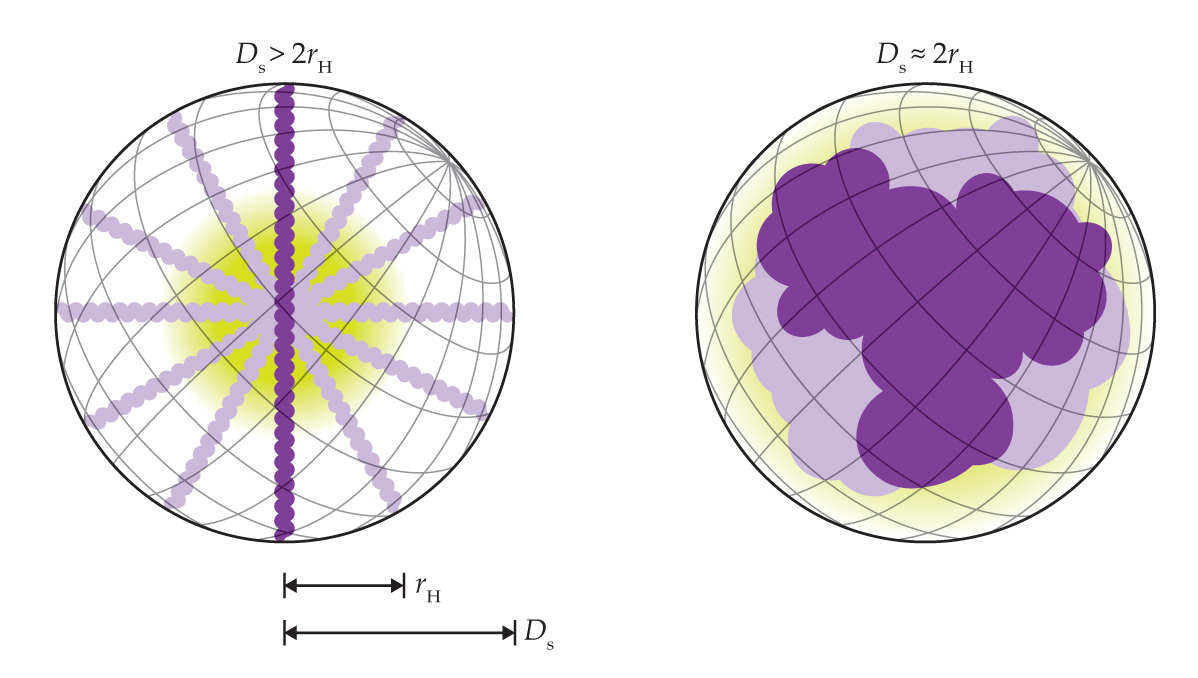
Although the researchers’ initial focus was on nanoparticles, they argued that the same principle could be used to trap single proteins and other biomolecules. “But I don’t know if anyone really believed us at the time,” says Krishnan. The application of the technique to molecules, however, ended up going in a different direction. Krishnan realized that if she could design the traps not to be so deep that they’re inescapable, she could learn useful information from how long it took the molecules to escape. 3
Escape probability per unit time is exponentially related to the energetic depth of the trap, which in turn is proportional to the effective amount of charge on the molecule. By measuring average escape times, the researchers could get a sensitive look at how much charge molecules carry.
One can measure escape times easily and accurately by fluorescently labeling the molecules and watching them under a fluorescence microscope. Pockets light up when they have molecules in them, and they go dark when empty. Meanwhile, molecules that are in transit between pockets don’t show up on the microscope image because they’re moving too fast. Escape is a stochastic process, so the researchers need to observe many events to calculate the average escape time—which is typically in the tens of milliseconds—but they can do that with a minute’s worth of data.
Because molecules don’t carry charge randomly, escape-time electrometry, as Krishnan and colleagues called it, gave some useful information about structure. DNA, for example, carries a fixed amount of charge per base pair, so DNA segments of different lengths can be distinguished by their escape times. 4 “We spent about a decade on this obsession with charge,” says Krishnan. But the technique could do much more: It could access structural information directly.
Two measures of size
The time it takes a molecule to escape from a pocket depends not just on its charge but on its size: All else being equal, bulkier molecules take longer to escape than compact ones do. But unlike the exponential dependence on charge, the size dependence sits in the prefactor. “We thought that the size measurements would be less sensitive,” says Krishnan, “so at first, they seemed less exciting.”
Moreover, to access size information at all, the researchers would need to eliminate the influence of charge, which would otherwise be overwhelming. They could do that by adding salt: Flooding the solution with ions blunts the repulsive force between the charged molecule and the charged walls. But it seemed like that would be sacrificing the technique’s greatest advantage, so for a long time, they never tried.
Krishnan credits her postdoc Xin Zhu with taking the eventual leap. “He’s an excellent experimentalist, and one day he said, ‘I’m just going to try it,’ ” she says. “And it worked—once, twice, and then there was no looking back.” It was only once they had the experimental data in hand that the researchers realized the real power of the size measurements: Bigger molecules are slower to escape for two distinct reasons, so the escape time probes two properties of molecular structure.
With the electrostatic repulsion having been screened out, it’s primarily entropy that keeps the molecules in the traps. Only a few of the many possible random trajectories lead toward the exit. So the first way that size affects escape time is through diffusion speed: Bigger molecules move more sluggishly, and they can make fewer attempts to escape per unit time. Escape time is therefore directly proportional to the hydrodynamic radius rH—also called the Stokes radius—which is defined as the radius of the sphere that diffuses at the same speed as the molecule does.
Figure 3.

DNA nanostructures of similar size but different shape can be distinguished through their escape times. (a) The tile and bundle structures each consist of 240 nucleotides. But as the height h1 of the exit is decreased, the bundle’s escape time shoots up. (b) From escape-time measurements on two chips with different h1 values, researchers can solve for the hydrodynamic radius rH and molecular-envelope diameter Ds of both molecules. (Figure adapted from ref.
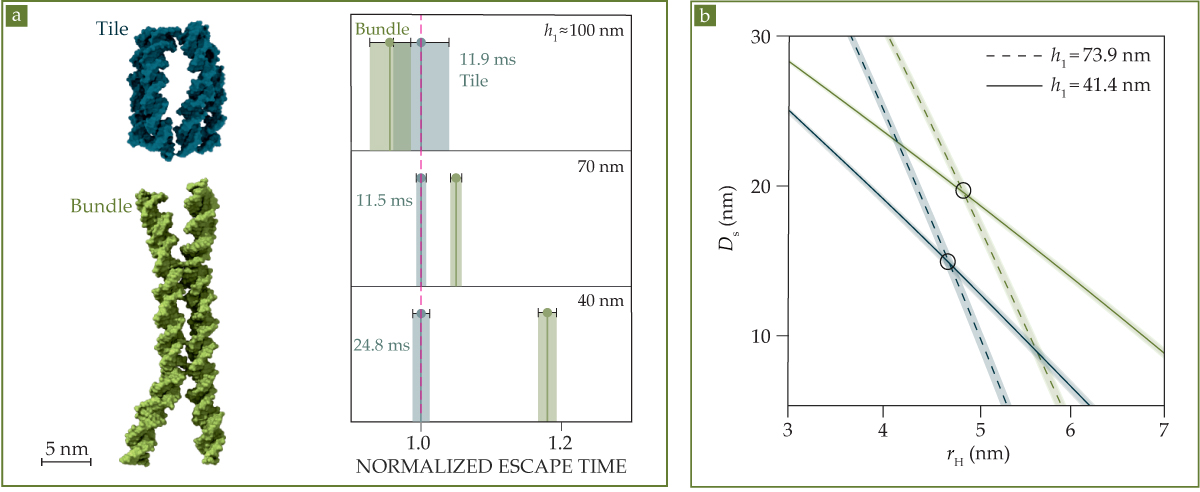
If that were all there is to it, it wouldn’t be very exciting. “There are lots of ways to measure the hydrodynamic radius,” says Krishnan. But it’s not enough for a molecule to diffuse to the edge of the trap; it must also fit through the exit. Specifically, the number of trajectories the molecule could take through the exit depends on the total clearance it has on either side. That clearance can be written as h1 − Ds, where h1 is the height of the exit, as shown in figure
The relationship between rH and Ds is shown in figure
The average escape time is just one quantity, so a single measurement isn’t enough to determine both rH and Ds. But by repeating the measurement on two or more chips with different h1 values, the researchers can solve for both structural parameters, and they can distinguish similar molecules. Figure
The difference becomes apparent as h1 is decreased, as shown in figure
The tile and bundle are known structures that were deliberately synthesized, and they don’t interconvert. But the same measurements could be used to characterize molecules that switch between different shapes and sizes in unknown ways: proteins toggling between two different structures, for example, or enzymes binding and unbinding from a molecular substrate. “With a mix of two different escape times, we’d have a biexponential distribution,” says Krishnan, “but as long as the interconversion time is long compared to the escape time, we can distinguish a molecule flip-flopping between two states by following it in time through the landscape of traps.”
Shining brightly
The technique is intended for proteins and other biomolecules, but it also works on organic molecules with as few as a couple of dozen carbon atoms. In that regime of relatively small molecules, the escape-time measurements have sufficient resolution to distinguish molecules that differ by one or two atoms.
A limitation of the measurements—especially significant for smaller molecules—is that the molecules of interest need to be fluorescently labeled, because that’s how the researchers detect whether a molecule is in a pocket or not. For the proof-of-concept experiments on smaller molecules, the bulk of the molecule was the fluorescent dye itself, so it’s not yet possible to apply the technique to arbitrary organic molecules.
“But that’s not a theoretical limitation,” says Krishnan. “Broadly, there are two ways to optically detect a molecule in solution: either through fluorescence or because the molecule itself scatters light. We can’t do these experiments with scattered light yet, but maybe in the future, the technology will have advanced enough to enable label-free operation. All the physics of the measurement remains the same in either case.”
The ability to measure Ds was a surprise, and the researchers look forward to more surprises in store. “The moment you have a new technique, I like to think you have very little predictive power in where it’s going to go,” says Krishnan. “We know that molecular conformation is very important, and that it’s tied to interactions, chemical affinities, and reactions. But the hope is that something completely unexpected comes out of this.”
This article was originally published online on 18 June 2025.
References
1. X. Zhu et al., Science 388, eadt5827 (2025).https://doi.org/10.1126/science.adt5827
2. M. Krishnan et al., Nature 467, 692 (2010).https://doi.org/10.1038/nature09404
3. F. Ruggeri et al., Nat. Nanotechnol. 12, 488 (2017).https://doi.org/10.1038/nnano.2017.26
4. M. Bespalova et al., Macromolecules 55, 6200 (2022).https://doi.org/10.1021/acs.macromol.2c00657
More about the authors
Johanna L. Miller, jmiller@aip.org




