A femtosecond laser pulse makes molecular bonds
DOI: 10.1063/PT.3.2868
For centuries scientists have controlled the fate of chemical reactions using macroscopic variables such as temperature, pressure, concentration, and pH. Since the advent of lasers, they have also increasingly exploited a microscopic variable—the internal structure of the reactants themselves. In that approach, a coherent electromagnetic field directly manipulates the wavefunctions of the reactants to steer the system’s dynamics toward some desired outcome.
With its high intensity and broad bandwidth, an ultrashort laser pulse is an ideal tool for the job: The multiple frequency components in its Fourier spectrum can simultaneously excite electronic and vibrational degrees of freedom of molecules. The pulses can also be precisely shaped by adjusting the phase, amplitude, and polarization of each spectral component to produce the quantum interference needed to constructively enhance one outcome of the reaction while destructively suppressing the alternatives. (See the article by Ian Walmsley and Herschel Rabitz, Physics Today, August 2003, page 43
Such coherent control, however, has been mostly limited to studies of processes like excitation and selective dissociation of single molecules. Controlling the reverse process of photoassociation—the light-induced formation of a molecule from independent atoms or precursor molecules—is a much harder task. The difficulty lies in the initial state of the reaction. A pair of colliding atoms can photoassociate only if they happen to be nearly the same distance from each other and moving with nearly the same relative speed as they would in the molecule. Because the range of interatomic distances, kinetic energies, and angular momenta in a gas of atoms can be huge, even at room temperature, such pairs are rare.
In 2011 a collaboration led by experimentalist Zohar Amitay (Technion–Israel Institute of Technology) and theorist Christiane Koch (University of Kassel in Germany) found that when a train of 70-fs laser pulses was fired into a hot, dense gas of magnesium atoms, each pulse produced molecules out of a small subset of atom pairs—about 10−4 of the entire irradiated ensemble. The pulses created Mg2 dimers by photoexciting the colliding atoms into superpositions of bound vibrational and rotational levels in an electronically excited molecular state. 1
Now, four years later, the collaboration has demonstrated that the new bonds can be coherently controlled and stabilized—at least in the molecules’ excited state—by adjusting the shape of each pulse during the brief time it shines on the atoms. 2 The achievement is an important milestone in the march toward a richer and more muscular photoinduced chemistry.
An IR control and a UV signal
Magnesium is exceptionally well suited for photoassociation studies. At the 1000-K temperature of the group’s experiments, hardly any molecules populate the ground state; so small is the attractive well that it’s not even perceptible at the energy scale of the molecular potential energy diagram shown in figure 1. The wells of electronically excited states of the molecules, by contrast, are deep and conveniently accessible with the near-IR photons from a titanium-doped sapphire laser. The hot gas of molecules is also a simple enough system that Koch and her theorist colleagues were able to go beyond semiempirical methods and calculate from first principles the energy states and dynamics produced by the laser’s intense electric field.

Figure 1. Shaping molecule formation. In this potential energy diagram of magnesium dimers, two IR photons from a femtosecond titanium-doped sapphire laser excite a pair of colliding atoms into a superposition of molecular vibrational levels (pink band) within the lowest excited electronic state (red curve). When the IR pulse is positively chirped, its instantaneous frequency increases with time (see inset), and Raman transitions lower the vibrational energy (black dashed lines) of dimers in the lowest excited state. A final photon then promotes the population in the lower vibrational states into higher-lying excited states (blue band), whose UV fluorescence to the ground state signals the presence of molecules. (Adapted from ref.
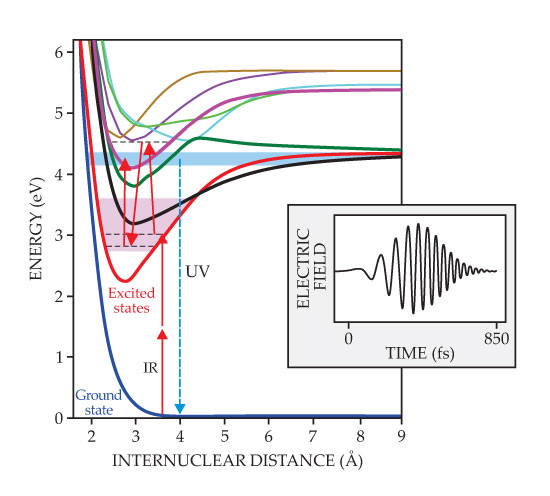
The first two IR photons in an intense laser pulse populate the lowest-energy excited state with freshly made dimers. A subsequent photon then elevates them to higher states, whose symmetry, unlike that of the first excited state, allows them to fluoresce to the ground state by emitting UV light. Because the molecules are created in the IR but detected in the UV, the signal is essentially free of background noise—an important feature given the low probability of photoassociation.
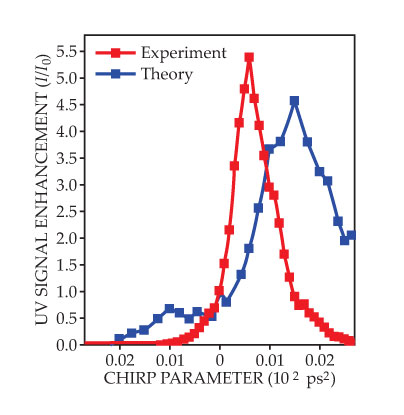
To shape the laser pulses in the new work, Amitay, Koch, and colleagues sent them through a liquid-crystal spatial light phase modulator. Now used routinely, such devices apply a computer-controlled electric signal to change the effective refractive index of the liquid crystal at each spatial location, thereby manipulating each frequency of the light pulse in a point-to-point mapping of space to frequency. The researchers found that a positive chirp—a pulse whose instantaneous frequency increases with time—boosted the yield by as much as a factor of five compared with an unshaped pulse, whereas a negative chirp suppressed it, as shown in figure 2. (For more on pulse chirping, see the article by Gérard Mourou, Christopher Barty, and Michael Perry, Physics Today, January 1998, page 22

Figure 2. The UV intensity from the fluorescence of magnesium dimers depends on the excitation laser pulse’s chirp parameter, which determines the pulse duration and rate at which the pulse’s instantaneous frequency increases or decreases. Positive chirps enhance the signal intensity I, or yield of detected dimers, relative to that (I0) from an unshaped pulse—an indication of the chirp’s stabilizing effect on newly formed molecules. Negative chirps, by contrast, suppress the yield by raising the molecules’ vibrational energies. Reassuringly, theory reproduces that main feature of the experiment. The rightward shift in the theoretical optimum chirp stems from inaccuracies in the calculation of the potential energy curves of highly excited states. (Adapted from ref.
Learning curve
To explain the chirp dependence, the researchers constructed a model in which, following the photoassociation step, so-called resonant Raman transitions between the molecules’ excited states raise or lower the molecules’ vibrational energy in the lowest electronically excited state. The transitions, which exchange energy between the molecules and the light field, can modify the vibrational distribution because the energy of each photoinduced transition differs—increasing or decreasing with the instantaneous frequency of the electric field.
Figure 1, in which the chirp is positive, illustrates one of many possible sequences of Raman transitions. The reduction in the molecules’ vibrational energies stabilizes their bonds and leads to an increase in the UV emission signal. The energy-lowering process is limited by the intensity and bandwidth of the laser, though, and the signal eventually drops when the chirp of the pulse becomes too high. The drop is mainly due to the significantly reduced maximum light intensities associated with larger chirps, which stretch out the pulse duration to as much as several hundred femtoseconds.
Confronted with that limit, the collaboration further optimized the shape of their pulses by sending them through a closed-loop process guided by a learning algorithm. 3 The effect on signal enhancement from a trial set of shaped pulses is fed back into the computer guiding the spatial light modulator. The computer generates new designs for the pulse shape in consecutive experiments until, as Princeton University’s Herschel Rabitz puts it, “the molecules succeed in teaching the laser the best shape that controls them.” The optimization, which mainly added amplitude variations to the temporal pulse shape, bought the collaboration a 35% increase in the yield of detected dimers.
In the case of the bimolecular Mg reaction, Mg2 is the only possible product. And it’s not long-lived. A nanosecond or so after the light pulse ends, the excited states decay and dissociate into atoms again—with or without a preceding UV emission. Ultimately, the research goal is to coherently control a chemical reaction whose product can be bottled up and preserved in the ground state, say, or used as a reagent in a subsequent reaction. As Amitay and Koch express it, “the controlled bond making of one pulse could be followed by bond breaking in the newly formed molecule by a second, time-delayed shaped pulse.”
Attempts to experimentally control more complicated bimolecular reactions will be accompanied, no doubt, by the daunting theoretical challenge of predicting their more complicated energetics and designing properly shaped pulses. Fortunately, learning-control algorithms are just as applicable to complex systems as to simple ones—a feature that alleviates the burden on theoretical prediction. Every system, one could say, solves its own Schrödinger equation.
References
1. L. Rybak et al., Phys. Rev. Lett. 107, 273001 (2011). https://doi.org/10.1103/PhysRevLett.107.273001
2. L. Levin et al., Phys. Rev. Lett. 114, 233003 (2015). https://doi.org/10.1103/PhysRevLett.114.233003
3. R. S. Judson, H. Rabitz, Phys. Rev. Lett. 68, 1500 (1992). https://doi.org/10.1103/PhysRevLett.68.1500




