A family of two-dimensional conductors comes into bloom
DOI: 10.1063/PT.3.5250
True metals are rare among two-dimensional materials. Ordinary metals such as gold, when shaved down to atomically thin dimensions, cease to conduct electricity: Their structures deform in a way that breaks the degeneracy of their valence and conduction bands. Even graphene, the electrical conductor of choice for 2D circuits and electrodes, is merely a semimetal, not a metal. The material lacks a bandgap, but its valence and conduction bands touch at only a few discrete points, so the quantum states that contribute to charge transport aren’t as plentiful as they might be.
A notable exception is a class of materials called MXenes (pronounced “Maxines,” like the name) with the general formula Mn+1Xn, where M is a transition metal and X is carbon or nitrogen. With their trellis-like structure of alternating M- and X-atom layers, MXenes are slightly thicker than the one-atom-thin graphene, but not so thick to disqualify them from behaving two-dimensionally. And their sturdy scaffold of covalent chemical bonds is robust enough to resist the conduction-destroying deformation that afflicts ordinary metals.
First synthesized in 2011, MXenes have some extraordinary properties, including an exceptional ability to block electromagnetic waves. And with their combination of electrical conductivity, high surface area, and chemical versatility, they’re natural fits for applications in catalysis and energy storage. But despite their promise, they’ve been difficult to make cleanly and safely.
Now the University of Chicago’s Dmitri Talapin and colleagues are working to change that. They’ve developed two new MXene synthesis routes that could help streamline basic research and open the door to more practical industrial manufacture.
1
Figure
Figure 1.

Layers of the material Ti2CCl2, synthesized through a new chemical vapor deposition reaction, form hierarchically structured, flowerlike spheres. Directly synthesizing Ti2CCl2 and other MXenes from their constituent elements could pave the way for a broader campaign of basic research on the two-dimensional conductive materials. (Courtesy of Di Wang.)

“We’re not saying that this is ‘better’ than the existing methods,” says Talapin. “That’s yet to be seen. But it opens up new ways of studying what’s possible and what’s not possible. It’s a new way of thinking about MXenes.”
Strange routes
When the right bulk precursor exists, making 2D materials is easy. Graphite, for example, consists of layers of gra-phene that weakly cling together thr-ough van der Waals forces. The layers are readily peeled apart: As Andre Geim and Konstantin Novoselov showed in 2004, isolating graphene monolayers takes nothing more than a piece of ordinary sticky tape.
And it’s not just graphene. Other bulk van der Waals materials exist, and they, too, can be separated into their component layers using sticky tape or other tools that are nearly as simple. (See, for example, Physics Today, July 2017, page 16
But MXenes have allowed for no such treatment. They’re made from bulk materials called MAX phases, in which MXene-structured layers are interspersed with layers of a third element A, often aluminum or silicon. The A atoms are bound to their neighbors more weakly than the M and X atoms are bound to each other, but they’re still held in place by covalent or metallic chemical bonds, which are much stronger than van der Waals forces. The material preferentially fractures along the A-atom plane, but it can’t be mechanically broken up into MXene monolayers.
The MAX phases have been known since the 1960s as interesting materials in their own right: soft, electrically conducting ceramics whose layered structure gives them anisotropic properties. In the early 2000s, Drexel University’s Yury Gogotsi and colleagues showed that they could chemically remove the M and A atoms from a carbide MAX phase to obtain so-called carbide-derived carbon, which had useful patterns of porosity. 2 Then, in 2011, Gogotsi and colleagues found that bathing a MAX phase in hydrofluoric acid could remove just the A atoms. 3 Their result was flakes of Ti3C2, the first MXene.
“But the discovery came at the wrong time,” says Gogotsi. Geim and Novoselov had just been awarded the Nobel Prize (see Physics Today, December 2010, page 14
The uptake of MXenes remained slow outside the materials science and chemical engineering communities. Even Talapin, a chemist, calls his group a “rare example” of nonengineers working on the materials. “We came by strange routes in the beginning,” he says. His group’s expertise is in colloidal nanoparticles, and in 2016 he and his colleagues showed that with a molten salt as a solvent, they could make colloidal materials that weren’t possible in other liquids. “From there, it was an easy step to ask, ‘Can you make colloids in liquid metals?’” he says. “We arrived at MXenes as a model system to study van der Waals interactions in liquid metals, and then we realized that there was a huge opportunity in synthesis.”
Back to the future
For researchers unafraid of dangerous chemicals, the MAX-phase synthesis of MXenes is simple enough. “You just add hydrofluoric acid and stir,” says Di Wang, a PhD student in Talapin’s group and lead author on the new paper. But it’s limited in which MXenes it can produce. With MAX phases that have weaker-than-average M–X bonds, including most of those whose X atom is nitrogen, the HF doesn’t always stop at removing the A atoms: It breaks apart the M and X atoms too, leaving degraded MXenes or none at all.
Furthermore, the use of harsh acids makes it harder for MXenes to find their way out of the lab and into mass-produced consumer products. It’s not that the chemical industry shies away from HF—it’s used in producing Teflon, for example—but the hazard is a significant factor in any cost analysis.
Talapin and colleagues took the approach of synthesizing MXenes directly from their constituent atoms, rather than starting with a structure with too many atoms and etching away the excess. “There’s nothing we did that couldn’t have been discovered many years ago. The work that inspired us was published in 1986,” says Talapin, referring to a paper by Iowa State University’s John Corbett and colleagues, who created layered yttrium and zirconium compounds out of a few simple ingredients. 4
“We wanted to study the zirconium and yttrium reactions,” Talapin continues. “But the titanium MXenes are the most studied, so there’s the most information out there about whether we’re making them the right way or not.” In analogy with Corbett’s formulation, the researchers combined titanium metal, titanium tetrachloride, and graphite and baked them together at high temperature. The result was a van der Waals layered material with the formula Ti2CCl2, as shown in figure
Figure 2.

Atomic structure of a MXene. On the left is a ball-and-stick diagram of three layers of Ti2CCl2. On the right is an electron micrograph of the same structure showing the positions of the titanium and chlorine atoms. (Adapted from ref.
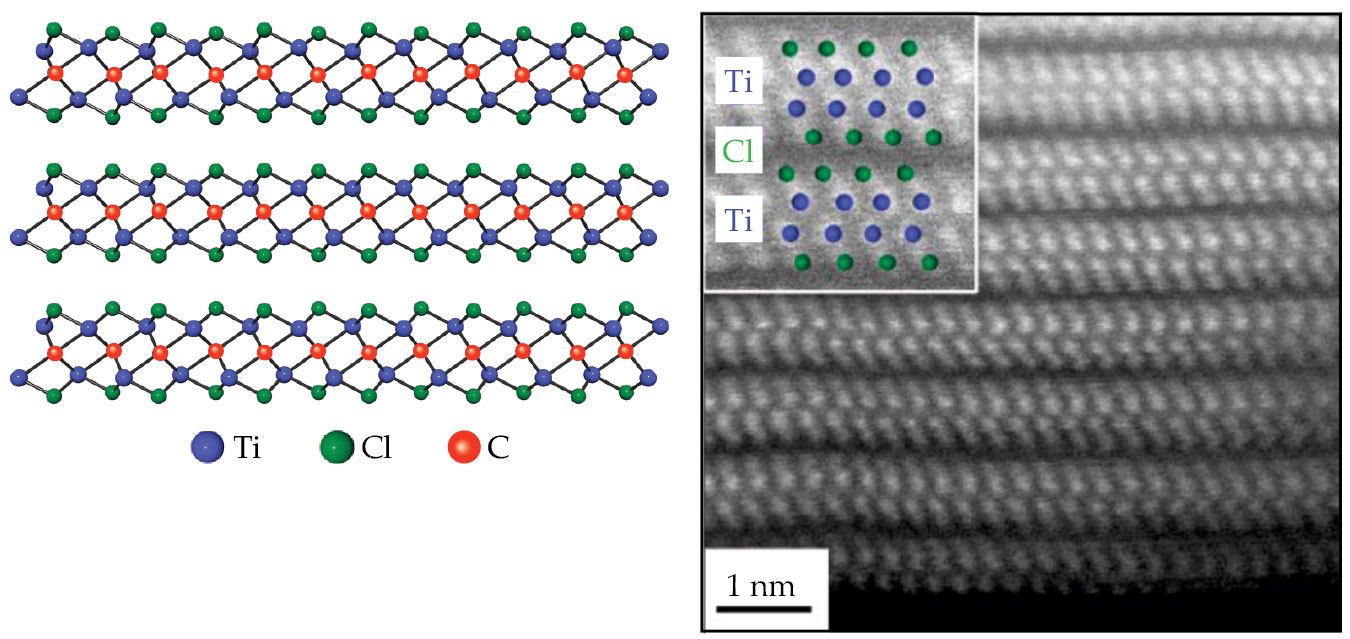
The presence of Cl atoms doesn’t mean that the structures aren’t MXenes. To the contrary, MXenes are almost always covered on both sides with some other atom or molecule, called a surface termination. In contrast to most other 2D materials, whose properties are radically disrupted by surface reactions, MXene surface terminations gently tune the MXene properties by adjusting the energy of the conduction-band electrons.
In fact, it’s notable that the synthesis produces Cl as the only surface termination. When HF etches away the A atoms from MAX phases, it leaves MXenes covered with an unpredictable mix of fluorine atoms, oxygen atoms, and hydroxyl groups. “From the MAX phases, we get MXenes with poorly controlled surface terminations when using aqueous chemistry,” says Gogotsi. “Talapin and colleagues’ direct synthesis is much cleaner in that regard.”
Runaway carpets
Two ingredients in the direct synthesis, titanium metal and graphite, are both solid at room temperature. (The third, TiCl4, is a liquid with a low boiling point.) Talapin and colleagues realized that they could make MXenes in a way that’s more compatible with device manufacture if they swapped graphite for methane as their carbon source, left Ti as the only solid reactant, and thereby gave the MXenes a single solid support on which to grow.
That type of process, called chemical vapor deposition (CVD), is widely used to produce materials in both two and three dimensions. But CVD synthesis of MXenes had never been demonstrated before. “So there weren’t many papers to help us or tell us what to expect,” says Wang.
Intuition would suggest that a CVD reaction should be self-limiting: When the reactants run out of available surface, the reaction should stop. But that’s not what happened. The MXenes first grew perpendicularly up from the Ti surface like a carpet, not flat on top of it like a laminate floor. Once the carpet covered the whole surface, it buckled upward, and the reaction kept going. The result was the spherical flowerlike structures shown in figure
The flowers aren’t just nice to look at. Because the MXene sheets are oriented perpendicular to the outer surface, they’re also ideal for applications in energy storage. To store and release energy quickly, batteries and electrochemical capacitors need high-surface-area electrodes that can hold large numbers of lithium and other ions. (See the article by Héctor Abruña, Yasuyuki Kiya, and Jay Henderson, Physics Today, December 2008, page 43
Strength in diversity
The sheer number of MXene structures is often touted as one of the family’s greatest advantages. 5 Between all the possible M elements, X elements, sheet thicknesses, and surface terminations, there are hundreds of possible MXenes. For MXenes that are solid solutions of two or more metal elements, there are countless more options.
The titanium–carbon MXenes are by far the most studied, so that’s what Talapin and colleagues focused on for their demonstration. But the researchers also showed that their synthesis schemes can produce many more MXene types, including several that have never been seen before, such as nitride MXenes that can’t survive the HF etching method.
What does the world need with so many MXenes? One answer that’s already been explored has to do with MXenes’ use as solid-state catalysts. When a surface facilitates a chemical reaction between atoms or molecules adsorbed onto it, the specific surface properties, such as the spacing between atoms and the availability of electron states, matter a lot. The more MXenes there are, the more reactions they can possibly catalyze.
Beyond that, both Talapin and Gogotsi opine that a large part of MXenes’ potential remains undiscovered, and the exploration could benefit from new scientific perspectives. In particular, the role of the surface terminations in tuning MXene properties creates an unusual interface between solid-state physics and molecular chemistry, with room for input from researchers in both fields.
“MXenes are metals that behave like semiconductors,” says Gogotsi, referring to their combination of conductivity and tunability. “By chemically modifying the surface, you can modulate the optical and electronic properties. There’s an exciting demand for the physics community to come explore, to check the existing predictions and make new predictions.”
“The engineering side is well on track,” says Talapin. “There are brilliant people working in this space, with lots of ideas of what MXenes can be used for. But as the field switches from simpler applications to more complicated ones, the diversity of properties will be more important. The next wave of discoveries will surely come from making MXenes more familiar to physicists and chemists, who can add chemical and physical rigor and deep physical insights. I see huge opportunities here.”
References
1. D. Wang et al., Science 379, 1242 (2023). https://doi.org/10.1126/science.add9204
2. Y. Gogotsi et al., Nat. Mater. 2, 591 (2003). https://doi.org/10.1038/nmat957
3. M. Naguib et al., Adv. Mater. 23, 4248 (2011). https://doi.org/10.1002/adma.201102306
4. S. J. Hwu et al., Inorg. Chem. 25, 283 (1986). https://doi.org/10.1021/ic00223a011
5. A. VahidMohammadi, J. Rosen, Y. Gogotsi, Science 372, 1165 (2021). https://doi.org/10.1126/science.abf1581
More about the authors
Johanna L. Miller, jmiller@aip.org




