Water in Earth’s mantle
DOI: 10.1063/PT.3.1476
Abundant surface water is the hallmark of our blue planet. The oceans contain some 1.4 × 1021 kg of it, and an additional 15% of that mass is stored in ice sheets, groundwater, lakes, rivers, and other surface reservoirs. 1 The volume of the water is a key element in Earth’s climate and habitability. To appreciate how, one need only ponder the marked differences between continental and maritime climates and how they might change if the oceans, with their enormous heat capacity, were to make up a much larger or much smaller fraction of Earth’s surface.
Yet surface water is only a fraction of Earth’s water inventory. Over the past 20 years, scientists have come to appreciate that vast quantities are stored in Earth’s interior—and perhaps those of other rocky planets as well—and that such water, widely distributed as point defects in the silicate minerals of the mantle, exerts a critical influence on the behavior of solid Earth. 2 , 3 Furthermore, geologic evidence suggests that over Earth’s history, the fluxes of water between the interior and surface—via volcanism and subduction—have been large. 4 Thus many Earth scientists now suspect that the dynamics of plate tectonics and perhaps also the maintenance of a habitable climate are directly related to the storage, influences, and fluxes of water deep inside Earth. These phenomena are known collectively as Earth’s deep water cycle.
Because mantle-derived magmas universally contain small amounts of water, researchers have long understood that Earth’s mantle must be slightly hydrated. However, most of the mantle consists of nominally anhydrous minerals (NAMs), such as olivine, (Mg,Fe)2SiO4; pyroxene, (Ca,Mg,Fe)2Si2O6; and garnet, (Ca,Mg,Fe)3Al2Si3O12. And for a long time, it was thought that the water resided in minor quantities of hydrous silicate minerals or in melts.
Beginning in the late 1980s, researchers realized that the hosts for water were chiefly the NAMs themselves, which accommodate hydrogen ions as trace defects. Experimental work has shown that those defects, even in tiny quantities, significantly affect the properties of the minerals and rocks of the mantle. 2 Water (or more precisely, hydrogen) controls such important phenomena as the locus of melting and viscous creep in the mantle. It also modulates transmission of seismic waves and electrical conductivity through the interior and therefore may be detectable by remote imaging. In short, water in the mantle is not simply a passive reservoir produced by larger-scale processes but one of the more important influences on Earth’s structure and dynamics.
A history of water
Many important questions about Earth’s deep water cycle pertain to Earth’s early history. Where did Earth’s water originate, and how did it become partitioned between interior and surface reservoirs? Was water originally contained in the interior and outgassed via volcanism? Or was it mostly in the atmosphere and slowly ingassed via processes such as dissolution into an originally molten planet and, later, subduction through a solid one? Was the deep water cycle established together with plate tectonics, or did one come first, perhaps enabling the other?
Earth formed from a sequence of violent accretionary collisions with objects ranging in size from dust particles to modest planetesimals to the Mars-sized planetary embryo thought to be responsible for the origin of the Moon. (See the article by Robin Canup in PHYSICS TODAY, April 2004, page 56
The extent of the loss of water and other volatiles to space during giant impacts may have depended on whether liquid oceans existed. The expansion of liquid to steam would have greatly increased upward accelerations generated by the impact and thereby enhanced atmospheric loss. Without liquid oceans, much of the protoatmosphere on the far side of the planet would have been retained during a giant impact. 5 If Earth was desiccated by the giant impact, it must have regained water and other volatiles through the accretion of a late “veneer”—the last few percent of Earth’s mass that was added by the arrival of planetesimals after the Moon-forming impact.
The thick steam atmosphere created by countless impacts contained much of Earth’s early water budget. In modern Earth, much of that inventory is stored in the interior. In Earth’s earliest history, though, the heat of impacts was sufficient to melt all or much of the planet, perhaps repeatedly. A fair portion of the steam probably dissolved into the mantle when it was nearly entirely molten. Some of that dissolved water was outgassed when the mantle solidified, because water’s solubility in magma is significantly greater than in NAMs.
Yet some water was undoubtedly trapped in the crystallizing silicates. The steam atmosphere soon condensed to form the early oceans, which are known from the most ancient mineral samples (zircons from the Jack Hills in Western Australia) to have existed at least as far back as 4.45 billion years—within 100 million years of Earth’s formation. 6 The resulting apportionment of water between the interior and the surface is uncertain, but it may have been critical to the subsequent development of the modern deep water cycle.
Reservoirs and fluxes
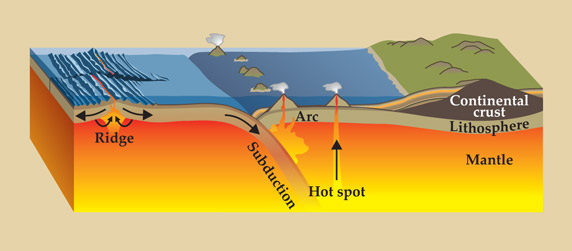
In the modern deep water cycle, partial melting of the mantle extracts water from it. As buoyant magma rises, the water outgasses into oceans and other surface reservoirs through volcanoes. The water is returned to the mantle by subduction, as pictured in figure 1. The fluxes of outgassing and ingassing water are governed by plate tectonics and simultaneously greatly influence the vigor of the tectonic processes through an incompletely understood feedback mechanism.
7
What’s more, the same cycling occurs for several other volatile compounds on Earth, as outlined in the

Figure 1. The modern deep-Earth water cycle is strongly coupled to plate tectonics. The flux of water from Earth’s mantle to its surface is governed by partial melting, which occurs mainly beneath mid-ocean ridges but also beneath “hot-spot” oceanic islands such as Hawaii. Mid-ocean ridges tap the upper mantle, which contains about 50–200 ppm water by weight, whereas hot-spot volcanoes tap deeper, more enriched portions of the mantle. Sediments and basalts on the ocean floor become hydrated from interactions with the oceans and return to the deep mantle by subduction—the sinking of old oceanic lithosphere into the interior. Some of that subducted water, however, is released from rock when it partially melts and returns to the surface via arc volcanoes, such as those of Japan and the Aleutian Islands. (Adapted from R. M. Hazen, R. J. Hemley, A. J. Mangum, Eos Trans. Am. Geophys. Union93, 17, 2012.)
That the volume of surface water closely matches the volume of the ocean basins and that, with comparatively minor fluctuations, it has done so for at least the last half of Earth history 8 are due to a balance between storage of water in the oceans and in the interior and a balance between outgassing and ingassing fluxes. But how much water is in the mantle? And what are the magnitudes of those fluxes between the deep and surface reservoirs?
Geochemical analyses of pristine volcanic glasses demonstrate that water is a universal minor component of the magmas that come from Earth’s mantle. The driest volcanic rocks on Earth are the basalts that erupt along mid-ocean ridges; the mantle that melts and eventually emerges as basalt contains between 50 and 200 ppm water by weight. If such concentrations prevail throughout Earth’s mantle, the interior contains at least 1020 kg of water—roughly 10–50% of the water on Earth’s surface. 1
However, judging from volcanic rocks from other localities, such as Hawaii and other oceanic islands, some mantle regions are more enriched, containing between 300 and 1000 ppm water. The proportion of Earth’s mantle represented by such sources is debatable, but if they are volumetrically dominant, then the mantle could contain as much as 2.5 times the mass of surface reservoirs. Thus a significant or perhaps even dominant fraction of Earth’s water resides in the interior.
As outlined in figure 1, the fluxes of water between Earth’s mantle and surface are controlled by volcanism and subduction. Volcanic eruptions occur primarily at diverging plate boundaries, where new oceanic crust forms at mid-ocean ridges, and at converging plate boundaries, where arcuate chains of volcanoes form. But important fluxes are also associated with volcanoes far from plate boundaries, such as the oceanic islands of Hawaii.
Correlations between the abundances of water and the rare-earth element cerium allow estimates to be made of the flux at mid-ocean ridges. Water and Ce behave similarly during partial melting of the mantle, but the abundance of Ce is known more accurately than that of water because Ce is easier to analyze and its abundance less susceptible to modification during and after volcanic eruptions. Ratios of H2O/Ce suggest that the flux of water from the mantle at ridges is 2 × 1011 kg/yr. Global fluxes of water vented at oceanic islands are more difficult to estimate but are believed to be a modest fraction of that.
Chemical reactions between seawater and the volcanic rocks that form the oceanic crust, together with accumulations of hydrated sediments on the sea floor, fix water molecules to the lithosphere—Earth’s rigid crust and uppermost mantle. The lithosphere subducts back into the mantle after residing on the sea floor for about 100 million years. Water is initially fixed into the crust as clays and other hydrous minerals. But as the subducting crust experiences higher temperatures and pressures, much of the water bound in the crust is liberated in a series of metamorphic reactions. Released water molecules incite partial melting in the overlying mantle and eventually emerge at the surface again in volcanic eruptions associated with island arcs such as Japan, Indonesia, and other provinces in the Pacific Ring of Fire. Some portion of the subducted water, though, remains bound in hydrous minerals or as trace defects in NAMs and is delivered to the deep mantle.
Based on researchers’ estimates of more than 1011 kg/yr for the global flux outgassed from Earth’s interior and 1021 kg for the planet’s total water budget, the time scale on which the deep water cycle operates is on the order of 109 years. The rather sensitive tuning of the cycling, such that the size of the surface reservoir remains relatively constant through much of Earth’s history, is due to the large reduction in rock strength from the presence of trace concentrations of H ions. Greater concentrations of water in the mantle promote more vigorous convection, which, in turn, enhances the flux of outgassed water. 7 The large influence of water on the vigor of mantle convection and plate tectonics competes with a similar influence of temperature: A hotter mantle supports faster convection, which, in turn, cools the interior more rapidly. The thermal evolution of the planet is therefore governed by the tradeoff between the effects of water and temperature on the vigor of plate tectonics. The magnitude of that feedback on Earth’s large-scale internal dynamics depends on the microscopic influence of trace H ions on the physical properties of NAMs in the mantle.
Microscopic considerations
Adding a small amount of H+ to a NAM is, in many ways, analogous to doping a semiconductor. Much as a small amount of boron significantly increases the electrical conductivity of silicon, a small concentration of H ions dramatically enhances the ionic conductivity of the mineral. The addition of H+ also increases the diffusivity of all the ions that make up the silicate structure. That increase, in turn, reduces viscosity.
How do minerals maintain charge neutrality as H ions are pumped into the mantle? The replacement of a Si4+ ion with an Al3+ ion plus an H+ is one possibility. However, such a mechanism would not account for the observed rapid uptake of H ions, which diffuse millimeters in a few hours at a temperature of only two-thirds of the melting temperature; in contrast, Al ions diffuse a few nanometers. Alternatively, the diffusion of H ions in iron-bearing silicate minerals can be charge compensated by a counter flux of holes—that is, the absence of electrons—in the reduction reaction Fe3+ → Fe2+ + hole. In that case, the solubility of H+ is limited by the concentration of ferric iron (Fe3+), which typically exists as roughly 1 part in 10 000 of the mantle’s ferrous iron (Fe2+).
Because H+ solubility can exceed 50 H per thousand Si ions, however, a third mechanism is required. The diffusion of H ions into an anhydrous mineral can be charge compensated by a parallel flux of Si, Fe, or Mg cation vacancies or possibly by a counter flux of oxygen interstitials. In any of those cases, the increase in vacancy or interstitial concentration on the Mg, Fe, Si, or O sublattices would account for the measured increases in cation and anion diffusivities in a water-rich mantle.
Although scientists generally agree about the importance of cation vacancies in charge compensating H ions squeezed into the lattice, the identity of those vacancies remains controversial. On the one hand, first-principle calculations for the mineral forsterite (Mg2SiO4, also known as iron-free olivine) indicate that the lowest-energy structure involves four H ions residing close to O ions and a Si4+ vacancy, 9 as illustrated in figure 2. The predicted O–H stretching frequencies for that structure are in good agreement with those measured in IR absorption spectra, as plotted in figure 3a.

Figure 3. (a) IR spectra taken from five olivine crystals, each heated to 1100 °C while subjected to different pressures. The sharp peaks above 3500 cm−1 correspond to OH-stretching bands at wavenumbers consistent with the presence of silicon vacancies. But the concentration of hydrogen, obtained by integrating the area under each absorption spectrum and plotted in (b) with additional data, increases linearly with water fugacity—a proxy for pressure that accounts for water’s chemical reactivity at high water pressure. The linear scaling is consistent with the presence of magnesium vacancies. Hydrogen ions undoubtedly substitute for both Si and Mg, but which vacancy dominates remains unresolved. (Adapted from ref.
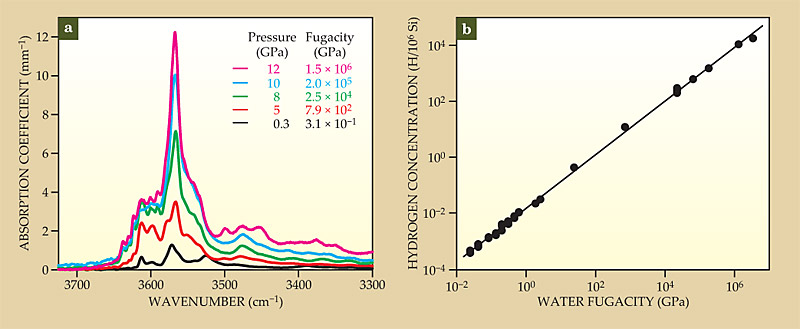

Figure 2. (a) The crystal structure of iron-free olivine, Mg2SiO4, also known as forsterite. Silicon ions sit at the middle of the blue tetrahedra; oxygen ions (red) sit at the corners. At a vacant Si site VSi, four H+ ions (white) can substitute for a single Si4+ ion. Alternatively, at a vacant Mg site VMg, two H+ ions can substitute for a single Mg2+ ion (yellow). (Adapted from ref.
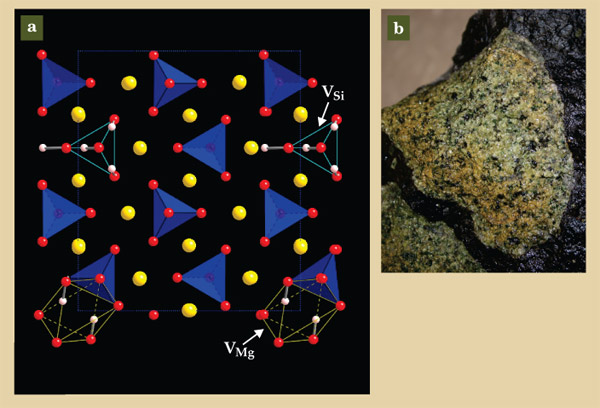
On the other hand, the measured linear dependence of H solubility in olivine on water pressure—or more precisely, on water fugacity, a parameter that accounts for water’s nonideal behavior with pressure—is consistent with a defect composed of two H ions associated with an Fe2+ or Mg2+ vacancy. That “defect associate” is also illustrated in figure 2 for Fe-free olivine, and the linear dependence of H concentration on water fugacity is plotted in figure 3b. The concentration of defect structures formed between four H+ ions and a Si4+ vacancy would increase as the square of water fugacity.
Water’s fugacity is a measure of its chemical reactivity in units of pressure. Water behaves as a nearly ideal gas up to a pressure of about 0.3 GPa for temperatures exceeding 1000 K. At higher pressures, corresponding to depths greater than a few kilometers, water is highly reactive (and thus soluble to H ions) and its fugacity far exceeds actual pressure, as noted in figure 3.
Two other observations favor Mg (or Fe) vacancies over Si vacancies as the primary charge-compensating defects. First, the change in molar volume as water is added to olivine is roughly the same as for MgO (or FeO), but half that for silicon dioxide. 10 Second, the ionic diffusivity, given by the product of the concentration of vacancies and their mobility, is in good agreement with that for Mg (or Fe) but orders of magnitude faster than that for silicon. 11
Macroscopic consequences
The point defects produced by the addition of H ions to silicate minerals have a profound effect on their physical properties. That influence was first recognized by James Blacic and David Griggs nearly 50 years ago in their investigation of the strength of quartz at high temperatures and pressures. 12 They noticed that quartz was substantially weaker if deformed while surrounded by talc rather than by an anhydrous material. Talc, or Mg3Si4O10(OH)2, releases water at temperatures above about 800 °C as it decomposes.
That water-weakening phenomenon has now been investigated in several other minerals, including olivine, pyroxene, and feldspar. Blacic and Griggs treated the phenomenon as an on–off event, as if minerals and rocks are weak under wet conditions but strong under dry ones. More recent investigations, however, have established that the strengths of NAMs and rocks systematically decrease as H concentration increases. 13
According to Blacic and Griggs, 12 water hydrolyzes strong, covalent Si–O bonds in NAMs via the reaction Si-O-Si + H2O ⇒ Si-OH-OH-Si. The movement of dislocations through a crystalline lattice becomes easier in wet quartz than in dry quartz because the presence of water precludes the need to break any Si–O bonds. Subsequent studies emphasized the role of H ions. Their rapid diffusion in a NAM indicates that protons, rather than the much larger hydroxyl ions, are the dominant water-derived point defects. 14 Thus water weakening might be more appropriately called proton weakening.
Other important physical properties of NAMs—including electrical conductivity, ionic diffusivities, and attenuation of seismic waves—are all influenced by the presence of protons. High-pressure, high-temperature experiments demonstrate a systematic increase in electrical conductivity with increasing water concentration. The dependence of conductivity on water concentration indicates that interstitial H ions are the primary charge carriers under wet conditions and dominate over holes, which are more important in a dry environment.
The diffusion rates of all the constituent ions also increase dramatically with increasing water fugacity. Indeed, the largest increase appears to occur for Si, whose diffusion in olivine at a water fugacity of 1 GPa and a temperature of 1200 °C is a factor of 1000 greater than it is under dry conditions at 1200 °C. Under the same wet conditions, O diffusion is enhanced by a factor of 10, and for such metals as Fe or Mg the diffusion is enhanced by a factor of 20. Those enhancements are generally attributed to an increase in the concentrations of vacancies or interstitial defects rather than in their mobility.
Seismic properties of rocks are also affected by relatively small amounts of water. The proton weakening of interatomic bonds is accompanied by a decrease in the mineral’s elastic stiffness. More significant is the influence of water on the attenuation of seismic waves. Processes such as the motion of dislocations—line defects in the crystal lattice—and the sliding of neighboring grains along interfaces that separate them dissipate the energy of a seismic wave and slow it down. The kinetics of those processes is enhanced in the presence of protons in crystalline grains and along grain boundaries.
Plate tectonics
Water may be critical for plate tectonics to develop as the stable expression of mantle convection on Earth. Analyses of the conditions required for the existence of plate tectonics identify two critical criteria that must be met. First, plate subduction must be initiated by the formation of a weak zone through the lithosphere. Numerical models of its response to loading by sediments or volcanic flows indicate that water weakening in the lower part of the lithosphere is necessary for a brittle fault nucleated at the surface to propagate all the way through the lithosphere. Second, a layer of low viscosity known as the asthenosphere is required below the lithosphere for stable plate tectonic behavior. 15 The origin of that low-viscosity zone is almost certainly linked to the presence of water in the mantle, as suggested in figure 4. Mantle rocks containing one H per thousand Si ions are more than two orders of magnitude less viscous than water-free mantle, a profound water-weakening effect. 13

Figure 4. Water and plate tectonics. Earth’s lithosphere, a stiff, high-viscosity lid about 70–100 km thick, overlays a soft, low-viscosity region, the asthenosphere, in which much of the deformation associated with mantle convection occurs. The lithosphere has high viscosity in part because it is dry; and the asthenosphere is weak because it contains more water—in the form of point defects—in its minerals, and perhaps because small amounts of melts rich in water and carbon dioxide are present. Horizontal arrows indicate relative velocities associated with convective flow: The lithosphere moves coherently—typically about 2–15 cm/yr—with no internal deformation, while considerable shear occurs in the asthenosphere. (Data from ref.
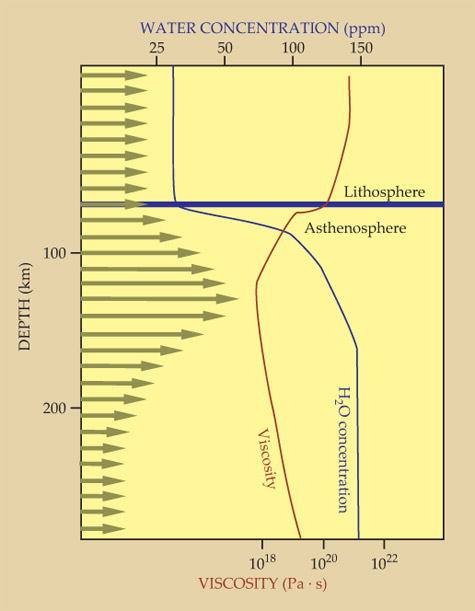
However, water may also have an indirect role on viscosity through its influence on the melting of mantle rocks. More specifically, water and CO2 in mantle rocks facilitate partial melting of the asthenosphere. 16 As little as 1% of the melt can reduce rock viscosity by an order of magnitude. 17 In addition, the removal of water from the rocks during partial melting increases the viscosity of the residual solid material in the lithosphere and thus further increases the viscosity contrast between lithosphere and asthenosphere. 2
In size Venus is often considered to be Earth’s twin, but in surface conditions the planets stand in marked contrast. NASA’s Magellan spacecraft mission revealed one striking difference: an absence of plate tectonics. The most likely reason for that absence is Venus’s high surface temperature, about 450 °C, which removes water from its lithosphere and prevents the localized deformation necessary to initiate subduction.
Clearly, the components of Earth’s deep water cycle, including both the persistence of water on the surface and its storage in the interior, are critical factors that affect Earth’s tectonic style. But the influence is also reciprocal: Plate tectonics controls the fluxes of water between the near-surface and deep reservoirs. The net flux can be monitored by what’s known as continental freeboard—in essence, a measure of the oceans’ volume based on the extent to which they have flooded the continental shelves over time. Studies of the record of sedimentary deposits indicate that sea level has changed very little in the past 600 million years and possibly over much of Earth’s history. Indeed, the record suggests a remarkable steadiness in the feedback between water cycling and tectonics. Understanding this feedback remains a key challenge.
Beyond water
Water is just one of several elements and volatile compounds that cycle between Earth’s surface and its interior and significantly affect the planet’s interior dynamics, surface geology, and habitability. Biologists, ecologists, and climatologists have long recognized the importance of the exchange of carbon between oceans, atmosphere, and shallow-surface environments. But on time scales from millions to billions of years, the deep carbon cycle has an equally profound influence.
Measured fluxes between the mantle and surface reservoirs suggest that terrestrial C, like water, cycles through the interior over some 109 years. Also like water, the surface reservoir of C is maintained by a balance between volcanogenic outgassing and subduction-driven ingassing. The ingassing includes C-rich sediments and C fixed into oceanic crust by chemical reactions between basalt and seawater. But as much as 90% of Earth’s C may be stored in the mantle. And judging by solubility measurements, it’s stored not in silicate minerals but in carbon dioxide–rich carbonate minerals (see PHYSICS TODAY, October 2003, page 21
On time scales of thousands to millions of years, volcanically emitted CO2 can have a critical influence on climate and the biosphere. During several periods in the Precambrian era—more than 500 million years ago—the planet experienced nearly global glacial conditions that shut down much of the near-surface C cycle. Earth emerged from that deep freeze thanks only to volcanic outgassing of CO2 from the mantle. Moreover, exceptional events, such as the eruption of so-called large igneous provinces, may release the gas in huge amounts, creating climatic crises and mass extinctions.
The early history of Earth’s deep C cycle is of particular interest. Carbon has a strong propensity to alloy with molten Fe, so a lot of the C delivered from space may have been sequestered in Earth’s molten core. How the large inventories in modern Earth and its near-surface reservoirs avoided that fate is unclear. Carbon may have been delivered as part of a late veneer, after the core had already formed. What’s more, because CO2 and other C-bearing vapors have very low solubilities in magmas, virtually all the C that was initially delivered to Earth and that escaped sequestration to the core should have ended up in the atmosphere rather than in the mantle. The thick C-rich atmosphere and its demise may have had a violent influence on Earth’s early climate that led, alternately, to very hot (tropical) and very cold (glaciated) conditions. Yet today, the inventory of C in the mantle far exceeds that locked in sediments and rocks of the crust. No one knows whether the reversal came about early on or developed over billions of years of plate tectonics. The answer may lie in a deeper understanding of how C behaved during the magma-ocean stage of Earth’s evolution.
Other key volatiles such as sulfur, nitrogen, and phosphorus, all critical ingredients for life, have their own deep cycling. In fact, much more terrestrial S resides in Earth’s mantle than in near-surface reservoirs, and an even larger quantity may be found in the core. Likewise, P may be stored chiefly in the mantle and core; the availability of P on the surface to form such biochemically critical molecules as adenosine triphosphate is therefore controlled by the element’s behavior during Earth’s long-term geochemical differentiation.
The storage of N in the interior is poorly understood, but if the mantle has just 1 ppm N, then it contains a mass equivalent to the entire atmosphere; and even more N may reside in the core. What governs the net storage of N in the interior and how it affects atmospheric dynamics? That and other questions regarding the behavior of deep-Earth volatiles are ongoing research topics.
References
1. C. Lécuyer, P. Gillet, F. Robert, Chem. Geol. 145, 249 (1998). https://doi.org/10.1016/S0009-2541(97)00146-0
2. G. Hirth, D. L. Kohlstedt, Earth Planet. Sci. Lett. 144, 93 (1996). https://doi.org/10.1016/0012-821X(96)00154-9
3. Q. Williams, R. J. Hemley, Annu. Rev. Earth Planet. Sci. 29, 365 (2001). https://doi.org/10.1146/annurev.earth.29.1.365
4. M. M. Hirschmann, Annu. Rev. Earth Planet. Sci. 34, 629 (2006). https://doi.org/10.1146/annurev.earth.34.031405.125211
5. H. Genda, Y. Abe, Nature 433, 842 (2005). https://doi.org/10.1038/nature03360
6. S. A. Wilde et al., Nature 409, 175 (2001). https://doi.org/10.1038/35051550
7. P. J. McGovern, G. Schubert, Earth Planet. Sci. Lett. 96, 27 (1989). https://doi.org/10.1016/0012-821X(89)90121-0
8. P. G. Eriksson et al., Earth Sci. Rev. 79, 165 (2006). https://doi.org/10.1016/j.earscirev.2006.07.001
9. K. Umemoto et al., Am. Mineral. 96, 1475 (2011). https://doi.org/10.2138/am.2011.3720
10. D. L. Kohlstedt, H. Keppler, D. C. Rubie, Contrib. Mineral. Petrol. 123, 345 (1996). https://doi.org/10.1007/s004100050161
11. D. L. Kohlstedt, S. J. Mackwell, Z. Phys. Chem. (Leipzig)207, 147 (1998).
12. D. T. Griggs, J. D. Blacic, Science 147, 292 (1965). https://doi.org/10.1126/science.147.3655.292
13. S.–I. Karato, H. Jung, Philos. Mag. 83, 401 (2003). https://doi.org/10.1080/0141861021000025829
14. S. Demouchy, S. Mackwell, Phys. Chem. Miner. 33, 347 (2006). https://doi.org/10.1007/s00269-006-0081-2
15. M. A. Richards, et al., Geochem. Geophys. Geosyst.2, 1026 (2001).
16. M. M. Hirschmann, Phys. Earth Planet. Interiors 179, 60 (2010). https://doi.org/10.1016/j.pepi.2009.12.003
17. Y. Takei, B. K. Holtzman, J. Geophys. Res. 114, B06205 (2009). https://doi.org/10.1029/2008JB005850
More about the authors
Marc Hirschmann and David Kohlstedt are professors of Earth sciences at the University of Minnesota in Minneapolis.




