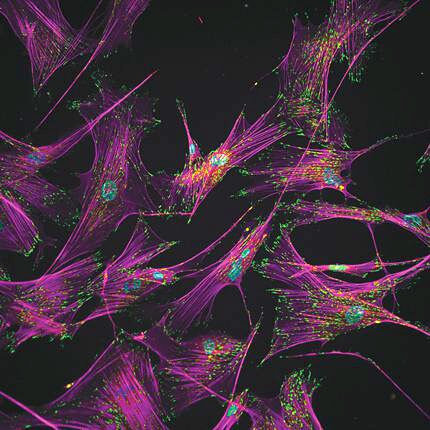
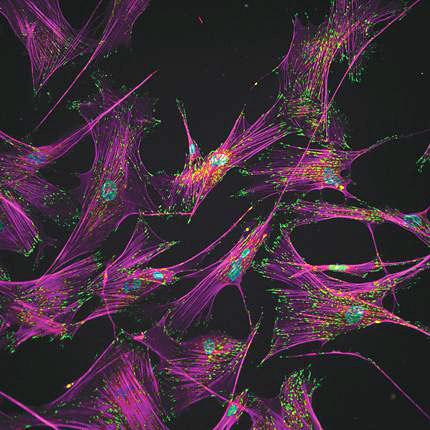
Separating stem cells by shear force
DOI: 10.1063/PT.3.1988
Last year’s Nobel Prize in Physiology or Medicine was awarded to John Gurdon and Shinya Yamanaka for the discovery that mature, specialized cells can be reprogrammed to be “pluripotent” stem cells, capable of becoming any type of cell in the body. That breakthrough opens numerous possibilities for regenerative therapies and for new studies of development and disease. Unfortunately, the reprogramming process, which typically involves reintroducing a handful of genes into connective skin cells called fibroblasts, has a notoriously low yield: rarely better than 1 cell in 50. And even then the induced pluripotent stem cells (iPSCs) have to be manually identified and extracted.
Andrés García and colleagues at Georgia Tech have now demonstrated a high-throughput microfluidic technique that in just a few minutes separates the iPSCs to purities approaching 99%, with a cell survival rate of 80%. The method exploits morphological changes that accompany the reprogramming. As befitting their structural role in the body, adult human fibroblasts adhere strongly to their environment. As shown here, actin filaments (purple) stretch tens of microns from each cell nucleus (blue) to numerous regions (green) that anchor the cell to its surroundings. In removing that specialized role, reprogramming also removes that specialized structure: The cells contract into spheres and adhere only about one-seventh as strongly to their surroundings. With such a different “adhesive signature,” the iPSCs could be robustly detached (and recovered downstream) by laminar flow through a microfluidic channel that the team placed over the cell culture. (A. Singh et al., Nat. Methods, in press, doi: 10.1038/nmeth.2437
To submit candidate images for Back Scatter, visit http://contact.physicstoday.org