Rare earths in a nutshell
DOI: 10.1063/PT.3.4397
As a class, the rare-earth elements comprise the 15 silvery-white metals, from lanthanum to lutetium, in the sixth row of the periodic table and the transition metals scandium and yttrium. Despite their name, most rare earths are not actually rare; nearly as many neodymium atoms reside in Earth’s crust as nickel atoms, for example. But neither do rare earths congregate in rich metal veins. They are instead widely distributed at low concentrations in mineral and coal deposits, which have made mining efforts difficult (see Physics Today, October 2018, page 22
The heterogeneous distribution notwithstanding, rare earths exert an outsize influence on our daily lives in the common products made with them, including motors, speakers, hard drives, and lasers. Materials that are exploited for their electrical, magnetic, or optical properties often consist of a few distinct types of atoms. Because of that compositional simplicity, their properties can be surmised, at least partially, from the location of their constituent atoms in the periodic table. This Quick Study explores the influence of the rare earths’ electronic structure on their properties and applications.
Lanthanide contraction
The filling and spatial extent of the outer electron (
Figure 1.

Lanthanide contraction and spin cycles. (a) The ionic radii of rare-earth R3+ ions and transition-metal M3+ ions are plotted as a function of atomic number
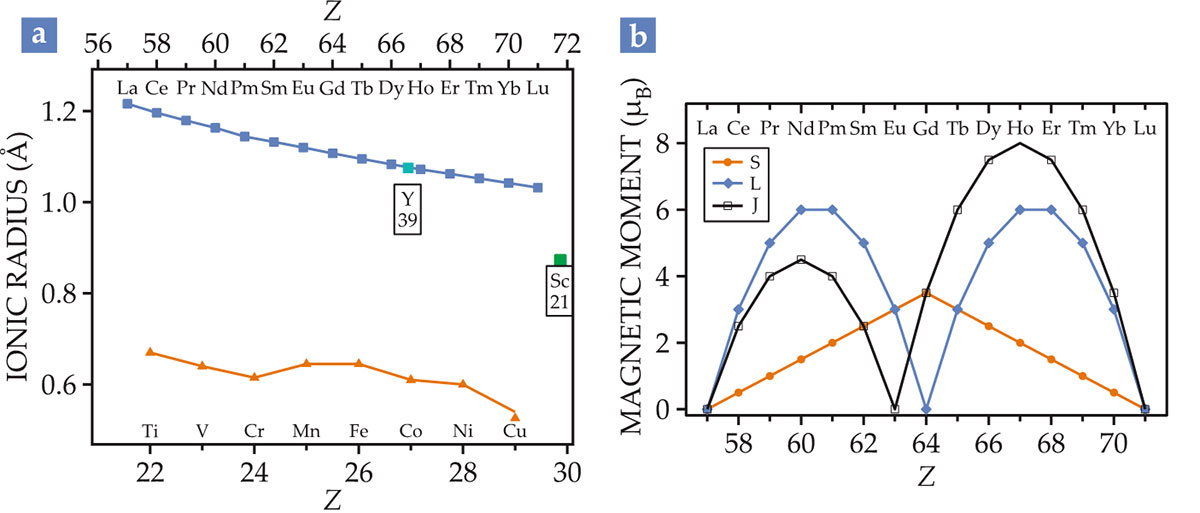
The ability to substitute one rare earth for another produces what’s known as chemical pressure on surrounding elements in a material. That pressure is either positive or negative, depending on whether the radius of the substituted element is smaller or larger than the native one. And it allows researchers to finely tune the properties of even a complex compound. A case in point: The magnetic phase of titanium oxide compounds (RTiO3) can be tuned from antiferromagnetic, with R ranging from lanthanum to gadolinium, to ferromagnetic, with R either yttrium or any element between holmium and lutetium.
Electrons in a rare earth occupy shells of either
By contrast, the
The magnitude of
When the spin–orbit coupling is large,
The multiplets of energy levels for electronic states on each rare-earth ion create a rich spectrum of light emission, most of it in the visible range of the electromagnetic spectrum. Moreover, the large magnetic moments of some rare earths and their anisotropy—the moment’s preferred direction in the crystal field—make the materials strongly magnetic.
Lasers and magnets
The lanthanide contraction, the identical nature of the outer electronic structure, and the spin–orbit coupling in the rare earths are exploited in a variety of applications. This Quick Study focuses on just two. Yittrium aluminum garnet (YAG) is a hard, durable, and transparent crystal widely used in lasers because of its high gain. The material’s lasing transition occurs between two energy levels of an Nd3+ ion in a Nd:YAG laser. In a YAG crystal co-doped with Er3+ and Ho3+, even more efficient lasing occurs because of the more favorable energy transfer between those ions.
Yittrium lithium fluoride is another popular host crystal for lasers. Because of their identical outer electronic structure and similar ionic size, Eu, Tm, and Yb make ready substitutes for Y3+ in the material. Other rare earths serve important functions in yet other laser systems. For example, carbon dioxide lasers produce IR light from transitions between molecular vibration levels. During high-power operation, some of the CO2 converts into CO, which often causes the laser to fail. Lanthanum strontium cobalt oxide is a rare-earth oxide that, when used as an electrode in the laser, catalyzes the conversion of CO back to CO2 and significantly extends the laser’s life span. Without La, the catalyst is unstable.
Magnetic applications benefit from the rare earths’ strong spin–orbit coupling. The interactions between the magnetic moments of electrons in partially filled
Popular rare-earth and transition-metal permanent magnets include samarium cobalt, neodymium iron boron, terbium iron, and gadolinium cobalt. Nd, Sm, Tb, and Gd have some of the largest moments of any rare earths (see figure
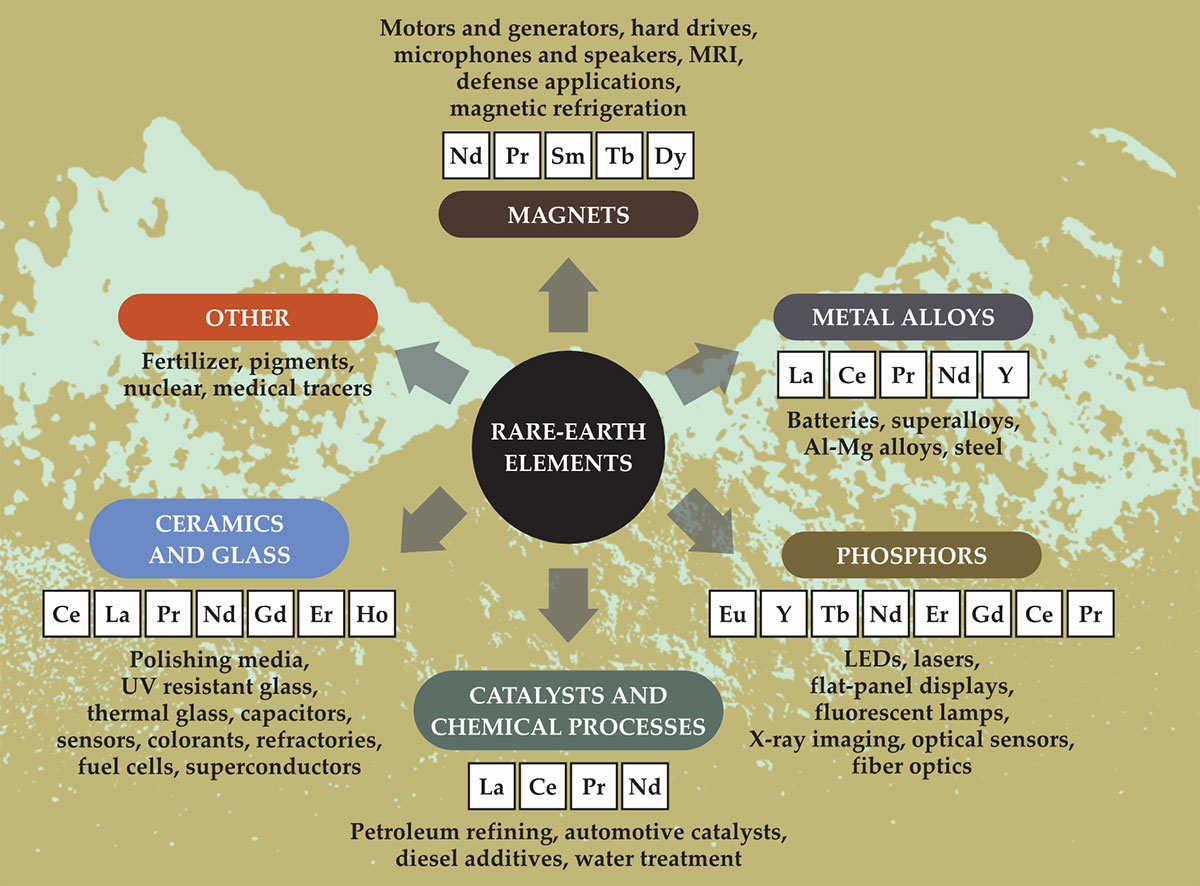
Figure 2.

Dozens of applications use rare-earth elements as key ingredients.
The bonding mismatch in a complex compound can cause a structural distortion. One can systematically tune that distortion or correct for it through rare-earth substitutions. In the orthorhombic RMO3 perovskites, for instance, the electron kinetic energy is proportional to the deviation of the bond angle M-O-M from 180°, where M represents a transition metal. A rare earth in the series from Lu3+ to La3+ widens the deviation from 145° to 165° in RNiO3. As a result, the perovskite changes from a metal to an insulator; and during that phase transition the strength of electronic correlations can be tuned over a broad range. Indeed, RNiO3 is a classic system for studying electronic correlations in solids.
Near the crossover from strong to weak correlations, exotic properties such as colossal magnetoresistance in the manganese oxides and high-temperature superconductivity in the copper oxides can be observed. And in those oxides, the lanthanide contraction is a key knob for tuning their electronic states.
References
► J. M. D. Coey, Magnetism and Magnetic Materials, Cambridge U. Press (2009).
► Handbook on the Physics and Chemistry of Rare Earths, vols. 1–43, Elsevier (1978–2013).
► J. H. L. Voncken, The Rare Earth Elements: An Introduction, Springer (2016).
► A. R. Jha, Rare Earth Materials: Properties and Applications, CRC Press, (2016).
More about the authors
Jianshi Zhou is a research professor in the department of mechanical engineering at the University of Texas at Austin, and Greg Fiete is a physics professor at Northeastern University in Boston, Massachusetts.






