Mirror asymmetry in life and in space
DOI: 10.1063/PT.3.3375
Take a look at your hands. Fingerprints and blemishes notwithstanding, your left and right hands probably look pretty much the same: four fingers, one thumb, a palm, and a back. Now try to overlay your left hand on your right and make everything line up. You can’t do it. Line up the palms so they’re both facing down and your thumbs point in opposite directions. Line up the thumbs, and your palms face one up, one down. Your hands are mirror images of each other, but they’re not superimposable, not identical. The term for that phenomenon is chirality, which, appropriately enough, is derived from the Greek word for hand, χειρ (kheir).
Molecules, like hands, can be chiral; the different-handed, mirror-image versions of a single molecule are called enantiomers. Enantiomers have identical physical properties. Their melting points coincide, as do their boiling points. They have the same spectral fingerprints in the frequencies at which they absorb light. And just as your left and right hands interact identically with nonchiral objects—think, for example, of gripping a baseball—chiral molecules interact identically with nonchiral molecules.
On the other hand, imagine trying to put a left-handed glove on your right hand or how awkward you’d find it if someone tried to shake your right hand with their left. Because your hands are mirror images, they interact differently with other chiral objects. Chiral molecules, too, share that property. For example, consider the molecule carvone. The two enantiomers, designated R and S from the Latin rectus (right) and sinister (left), interact very differently with the chiral taste and smell receptors in your body. R-carvone tastes and smells of spearmint; S-carvone is the principal component of the oil in the caraway seeds that give rye bread its distinctive flavor.
Many of the molecules essential to life are chiral. Among them are sugars; the nucleotides found in our DNA and RNA; and amino acids, which are the building blocks of proteins.
An evolutionary mystery
Every living organism on Earth has only left-handed amino acids. (An elaborate set of chemical conventions determines which enantiomer is designated “left-handed.”) That exclusivity, dubbed the homochirality of life, extends beyond amino acids to just about every chiral molecule essential for life, and with good reason. The famous double-helix structure of DNA, for example, is possible only if all the constituent nucleotides are homochiral. The resulting helix, which is itself chiral, interacts only with specific enantiomers of proteins and other chiral entities.
But why does life use left-handed amino acids? After all, the right-handed amino acids are identical in their physical properties. The answer is that no one knows. Certainly, it is evolutionarily advantageous for life to employ homochirality, which enables living organisms to build useful large-scale chiral structures such as the double helix. But the origin of homochirality and the utilization of one enantiomer over the other are among the great unanswered questions in the study of life’s origins.
A look back in time
Scientific consensus is that if a sufficient enantiomeric excess (ee) existed in life’s primordial molecular reservoir, that initial ee would almost certainly push life toward the extreme enantiomeric bias of actual living beings. Many theories have been put forth as to how and when such an ee might have been generated on early Earth. Meteoritic samples, however, hint at an even more ancient origin.
In 1969 a meteorite struck just outside of Murchison, Australia. Studies have shown that many of its amino acids have an excess of left-handed enantiomers, sometimes of more than 10%. Astrophysicists have determined that meteorites like the one that hit Murchison date back to about the time of our solar system’s formation. Moreover, the source of their molecular material can be traced to the cloud of gas and dust from which our solar system formed. If a reasonable mechanism exists for generating an ee in that primordial cloud, the origins of life’s enantiomeric bias could be linked to processes that occurred billions of years ago, before the solar system existed.
Perhaps the most plausible process for the creation of an ee in the solar system’s parent molecular cloud is via interactions with polarized UV radiation. Both left and right circularly polarized light are chiral, and chiral molecules will interact differently with the two polarizations. As a result, a small but measurable (in the laboratory!) amount of one enantiomer will be preferentially destroyed, so the other enantiomer will appear as an ee. We know that the interstellar medium contains circularly polarized light, but we have not been able to test the preferential-destruction pathway to ee; for one thing, until recently a chiral molecule had never been detected in space.
Hunting a complex molecule
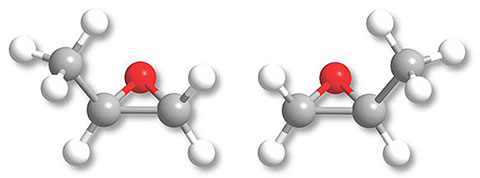
By interstellar standards, even the simplest chiral molecules are complicated, which makes their detection via radio astronomy challenging. As molecules become more complex, their rotational spectra—the signatures observed in the search for new molecules—not only become more involved, they also generally become fainter and harder to detect. One chiral molecule stood out to us as a good target for interstellar searches because it is relatively simple and because conditions in the interstellar medium are favorable for its formation. That molecule, shown in figure 1, is propylene oxide.

Figure 1. Enantiomers (mirror-image molecules) of propylene oxide. In these diagrams, hydrogen atoms are white, carbons gray, and oxygen red; the R enantiomer is the one on the left. Notice that aligning the methyl (CH3) groups would cause the oxygen atoms to point in opposite directions, whereas aligning the oxygen atoms would cause the methyl groups to be misaligned.
An excellent place to look for new molecules in the interstellar medium is near the center of the galaxy, in the star-forming region Sagittarius B2(N). With the 100-m-diameter Robert C. Byrd Green Bank Telescope, the Prebiotic Interstellar Molecular Survey (PRIMOS) project has observed large portions of the microwave spectrum in the direction of that region. As researchers working with PRIMOS, we found two of the rotational transitions of propylene oxide we were looking for. Unfortunately, the third, at about 12 GHz, was obscured by RF interference from orbiting satellites.
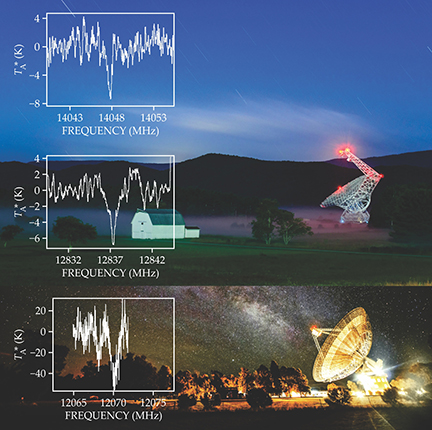
To confirm our detection and observe that elusive transition of propylene oxide, the PRIMOS team traveled to the 64-m-diameter Parkes telescope in Australia, where the offending satellite broadcasts were not present. We found the third, confirmatory transition of propylene oxide (see figure 2), and reported the detection of the first chiral molecule in the interstellar medium.

Figure 2. Spectra of propylene oxide. The two upper spectra were observed with the Robert C. Byrd Green Bank Telescope, shown in the background. The lower spectrum was taken with the Parkes telescope, again shown in the background. The specific definition of the background-subtracted temperature TA∗ is not essential here; the key point is that negative values represent absorption of light and positive values signify emission above background values. (Green Bank Telescope photo courtesy of Brett McGuire; Parkes telescope photo courtesy of CSIRO.)
Our observations of propylene oxide do not say whether, in fact, an ee of the molecule is present in Sagittarius B2(N). To answer that question will require precisely calibrated and extremely sensitive polarization observations of the absorption signal. If an ee is found, astrochemists can attempt to test theories about the conditions that gave rise to it. Then, given what we know of the initial conditions of our solar system’s primordial cloud, we can perhaps draw conclusions about the generation of an ee there and its implications for life on Earth.
References
1. M. H. Engel, S. A. Macko, “Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite,” Nature 389, 265 (1997). https://doi.org/10.1038/38460
2. J. Bailey et al., “Circular polarization in star-formation regions: Implications for biomolecular homochirality,” Science 281, 672 (1998). https://doi.org/10.1126/science.281.5377.672
3. S. Pizzarello, T. L. Groy, “Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective,” Geochim. Cosmochim. Acta 75, 645 (2011). https://doi.org/10.1016/j.gca.2010.10.025
4. B. A. McGuire et al., “Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O),” Science 352, 1449 (2016). https://doi.org/10.1126/science.aae0328
More about the authors
Brett McGuire is a Jansky Postdoctoral Fellow with the National Radio Astronomy Observatory in Charlottesville, Virginia. Brandon Carroll is a graduate student at Caltech in Pasadena.






