Making soft and reconfigurable electronics with liquid metals
DOI: 10.1063/PT.3.5260
Gallium has a melting point of 30 °C, which is low enough that body heat alone can melt it. The element’s melting point can be lowered further by dissolving other metals to form gallium-based alloys—such as eutectic gallium indium (EGaIn)—which are liquid at room temperature. Those alloys are referred to as liquid metals, or simply LMs. (See the article by Michael Dickey, Physics Today, April 2021, page 30
LMs are similar to water in some respects. For example, both water and EGaIn have similar viscosities, of 2.0 mPa·s and 1.0 mPa·s, respectively. They also both expand when they freeze. But water boils at 100 °C and has a vapor pressure of 2.33 kPa, whereas gallium boils at 2400 °C and has an extremely low vapor pressure, which practically eliminates inhalation concerns during its handling or processing. Additionally, EGaIn has metallic thermal and electrical conductivities that are orders of magnitude larger than water. Those attributes combined with EGaIn’s low toxicity make the alloy an attractive material for soft, stretchable, and wearable electronics.
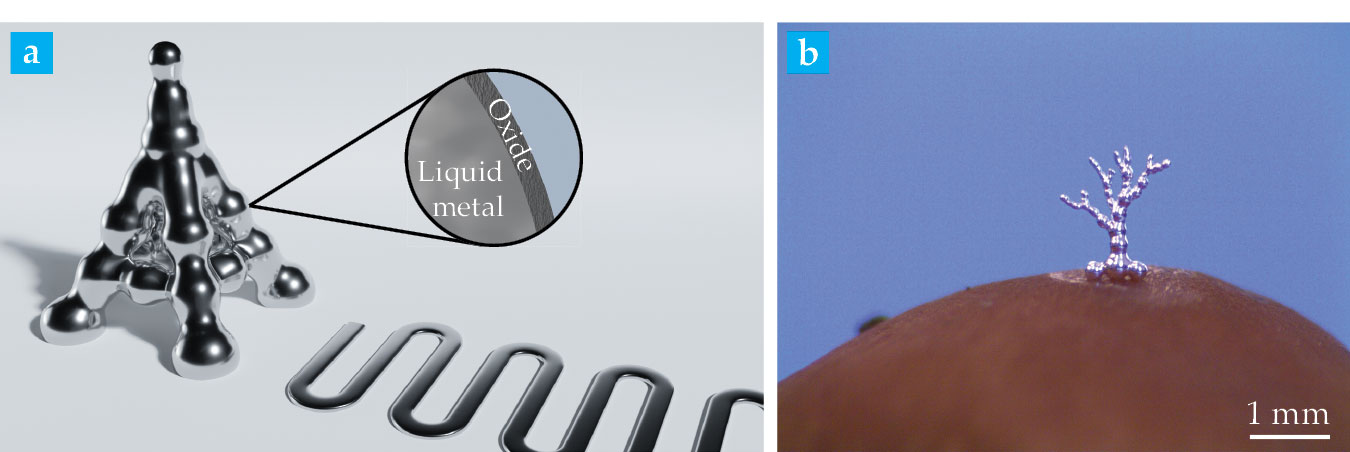
Advanced electronic applications such as interconnects, electrodes, and antennas require metals to be patterned into precise and stable structures. But controlling and stabilizing the shape of liquids is difficult because they tend to flow in response to force. Consider, for example, how difficult it is to create a stable cylinder of water without a container. Fortunately, gallium-containing LMs react spontaneously with oxygen in the air to form an oxide. That nanometers-thick oxide skin encases the LM and stops it from freely flowing, a fundamental difference compared with most other liquids. Water droplets in contact with each other simply merge into one larger droplet, but LM droplets can be patterned and stacked into three-dimensional structures, as shown in figure
Figure 1.

Liquid-metal structures. (a) Two- and three-dimensional patterns are stabilized by a thin oxide skin that forms when gallium or gallium-based metal alloys react with air. (Courtesy of Luke Cunningham.) (b) Three-dimensionally printed droplets of liquid metal are stacked on the surface of a mushroom. (Courtesy of Collin Ladd.)
Another property of LMs is their ability to significantly change their interfacial energy by a simple electrochemical reaction. Whereas surface tension typically refers to the tension of a fluid relative to a surrounding vapor phase, interfacial tension broadly encompasses the tension of fluids in contact with other materials. Of the elements that are liquid near room temperature, Ga has the largest surface tension, at 708 mN/m. EGaIn’s surface tension is slightly lower at 624 mN/m, still nearly nine times that of water. Such large tensions cause LMs to bead up into droplets in the absence of oxide. The most common way to lower tension is with surfactants, such as soap, but they’re not very effective. And once such molecules diffuse to the interface of a liquid, they are hard to remove.
In contrast, the interfacial tension of metals can be controlled electrochemically in an aqueous electrolyte. A reducing potential of −1 V removes the surface oxide by converting it to metallic EGaIn. Once the oxide is removed, the metal is left bare and has a high interfacial tension. It therefore beads up to minimize its surface energy.
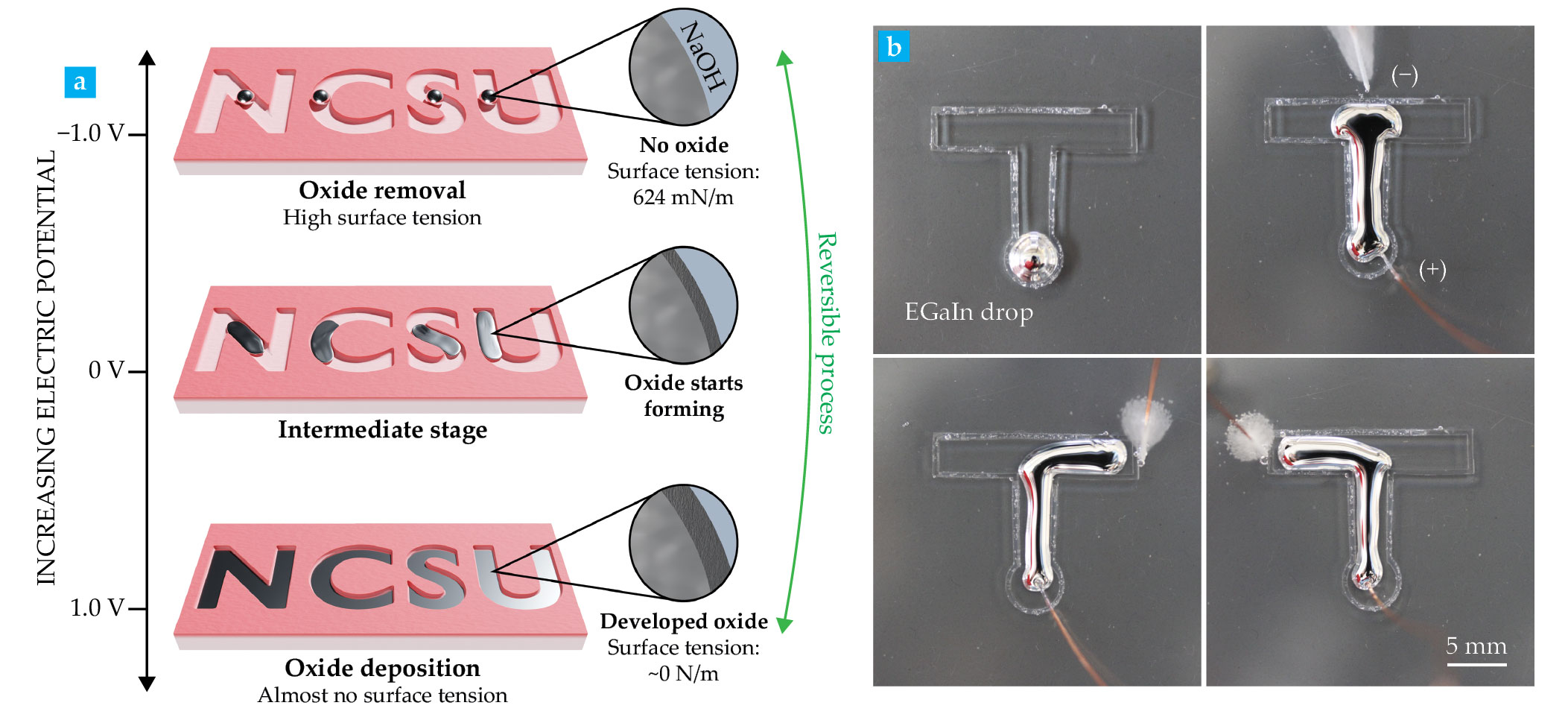
The effects of electrochemical reduction on the interfacial tension of LMs can be reversed with electrochemical oxidation. To demonstrate the effect, we applied a modest positive potential of 1 V on EGaIn immersed in a sodium hydroxide electrolyte. The potential drives the oxidation of the metal’s surface and lowers the interfacial tension of the LM from 624 mN/m to nearly 0 mN/m.
Altering the tension of a metal droplet in an electrolyte by gathering charges at the interface is a widely known phenomenon called electrocapillarity. It can’t, however, explain why the interfacial tension of LMs drops so much during electrochemical oxidation. Under those conditions, liquid streaming from a nozzle emerges as a wire rather than as the typical spherical droplet. One possibility is that the oxide species deposited electrochemically acts like a surfactant that resides between the metal and electrolyte. Another possibility is that the oxide itself exerts compressive stresses that counteract interfacial tension. The mechanism that lowers tension remains, for now at least, poorly understood.
By taking advantage of the ability to tune the tension of LMs using electrochemical reactions, we can manipulate the shape of the metal using modest electric potentials on the order of about 1 V. For instance, applying an oxidative potential causes the metal immersed in electrolyte to lower its interfacial tension, thus allowing the LMs to conform and fill a more complex geometry, as illustrated in figure
Figure 2.

Interfacial tension of liquid metals can be electrochemically controlled. (a) The tension of the liquid metal in a water-based solution increases with an applied reduction potential and is reversibly lowered with an applied oxidation potential. (Courtesy of Luke Cunningham.) (b) Variations in electric potential also control the shape and the direction of travel of metal droplets, as shown in this open T-shaped channel submerged in a sodium hydroxide solution. The different positions of the counter electrode—located outside of the T channel—dictate the flow direction of the metal droplet. (Adapted from M. R. Khan et al., Proc. Natl. Acad. Sci. USA 111, 14047, 2014, doi:10.1073/pnas.1412227111
Researchers have many opportunities and challenges to explore in using LMs for soft and stretchable devices. The interfacial chemical reactivity and stability, for instance, are not fully characterized for gallium-based alloys with standard metal contacts, such as nickel, silver, gold, and copper. But that characterization is critical if manufacturers are to successfully integrate Ga-based alloys in consumer electronics and if researchers are to identify the right barrier materials for the required electrical performance.
Although tremendous strides have been made in high-resolution printing and patterning, little research has explored the high-density LM interconnects that are needed to join electrical components. Some initial research has shown that LMs can be mixed as particles within other materials, such as filler within elastomers, to create composites with unique thermal, mechanical, and electrical properties. Depending on the desired application, LM composites can be tailored to produce highly stretchable and electrically conductive materials or made into electrically insulating but highly thermally conductive materials. LM researchers are particularly excited by thermal-management and thermoelectric applications in soft electronics.
In yet another application, physicists and other scientists are exploring how to use LMs for promoting reactions, including using them as a source for producing thin oxide layers. Research groups focused on those emerging research areas are striving to elucidate the fundamentals that govern the unique behavior and characteristics of LMs. With that understanding, they can harness the remarkable properties of LMs to create novel functional materials and devices.
References
► S. Liu, D. S. Shah, R. Kramer-Bottiglio, “Highly stretchable multilayer electronic circuits using biphasic gallium-indium,” Nat. Mater. 20, 851 (2021). https://doi.org/10.1038/s41563-021-00921-8
► J. Ma et al., “Shaping a soft future: Patterning liquid metals,” Adv. Mater. 2205196 (2023). https://doi.org/10.1002/adma.202205196
► M. R. Khan et al., “Giant and switchable surface activity of liquid metal via surface oxidation,” Proc. Natl. Acad. Sci. USA 111, 14047 (2014). https://doi.org/10.1073/pnas.1412227111
► N. Kazem, T. Hellebrekers, C. Majidi, “Soft multifunctional composites and emulsions with liquid metals,” Adv. Mater. 29, 1605985 (2017). https://doi.org/10.1002/adma.201605985
► A. Zavabeti et al., “A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides,” Science 358, 332 (2017). https://doi.org/10.1126/science.aao4249
More about the authors
Omar Awartani is a research scientist at Meta Reality Labs and an adjunct assistant professor in the department of mechanical and aerospace engineering at North Carolina State University in Raleigh. Michael Dickey is the Camille and Henry Dreyfus Professor in the department of chemical and biomolecular engineering at North Carolina State University.






