Janus particles
DOI: 10.1063/1.3177238
A friend of ours is shy and thinks a lot. His girlfriend used to tease him about how it was impossible to predict which side of him, the shy or the thoughtful one, would open his mouth when they met. She found that quirk more interesting than consistency, and her acceptance was, he thought, what made his shy life tolerable. They wouldn’t have met if they hadn’t both been good, contemplative students, but they wouldn’t have attracted one another so much if they hadn’t both been so shy. They each had two faces.
Inspired by our friend (our younger selves!), we want to understand what makes objects come together or repel each other. Textbooks emphasize the easiest case—how objects attract and repel one another when their orientation doesn’t matter. For example, gravity causes masses to attract and electrostatics causes like charges to repel, but those forces depend on separation, not on orientation. The physics of everyday life presents subtler options. Particles provide an interesting and useful paradigm: tiny Janus spheres that avoid one another if they face one way but cling together if they face the other direction. The bar magnets we played with as children are another simple system for which force depends on orientation, though the details differ: Magnetic interactions are so long ranged that they force the magnets to line up as strings. With particles whose interactions are softer—shorter in range than the particle size—a whole new spectrum of behavior may be observed.
The Janus principle
Janus particles are named after the Roman god Janus, depicted in figure 1. His two opposing faces peer to the past and the future, to the beginning and the end, to creation and destruction.

Figure 1. The two-faced god Janus. This sculpture is in Rome’s Vatican Museums.

Soap molecules and biological phospholipid molecules possess the awkward ambivalence symbolized by Janus. Their unhappy destiny is to be made up of polar head groups and hydrophobic tails bound together in the same molecule, fated to connect even though they hate one another. When added to water, their hydrophobic sides huddle together, protected by the polar head groups. DNA is also organized around the principle of ambivalence. Its elemental nucleotides, strung together to form a strand, contain one portion that is capable of forming hydrogen bonds with others and another part that is inert. The double helix is an intertwined pair of chains with the hydrogen-bond-forming parts pointed inward and the inert parts pointed outward. Proteins, too, join functionally different elements. They can be charged on one side and hydrophobic on the other, as are the soap molecules, whose like hydrophobic sides attract. Or they can be positively charged in spots but negatively charged in others, in which case opposites attract.
The above examples illustrate the Janus principle—the combination of incompatible elements in the same unit structure drives molecules to assemble spontaneously into some of the most useful and complex superstructures of everyday life. Soaps form micelles that are so effective for grabbing dirt and washing it from hands. Phospholipid molecules are a chief ingredient of cell membranes in biology. The same Janus principle enables DNA’s structure to store and protect information necessary to life and is behind proteins’ ability to become essential parts of organisms and to participate in virtually every intracellular process.
How to form Janus particles
Now imagine extending the principle to grains of sand large enough to see in an optical microscope but sufficiently small to be jiggled about by Brownian motion as if they had lives of their own. Particles of that size are ubiquitous in the world around us, as isolated entities and in applications. They are charming to study because one can look at them individually under a microscope and watch them interact with one another in real time. Physicists use them to probe the emergent properties of matter whose simple elementary interactions are well understood but whose complexity, such as crystallization and melting, results from more mysterious physics. Those beautiful studies can be made even more elegant and realistic with two-faced Janus particles.
Fortunately, the raw material is easy to come by. Start with colloids—particles larger than molecules but not more than a few micrometers in size—all of them identical or nearly so. The simplest are tiny spheres that all have the same size, but cubes, rods, ellipsoids, and more complex shapes are also possible. If one lines them up against a wall and sprays them with a chemical, the result is two faces: the coated side and the uncoated one that was protected because it faced the wall. Particles can also be made to line up at liquid-liquid interfaces of emulsions. (Think of microdroplets of vinegar surrounded by oil in a well-mixed salad dressing.) As emulsions pack a large surface area into a small volume, they enable researchers to produce large quantities of Janus particles by chemically modifying the particles from each liquid selectively. Other techniques will also produce Janus particles, and clever chemists are busily inventing new ways to create them.
Controlling the Janus balance, the relative area of the two sides, is just as important as placing anisotropic chemical makeup onto a particle. A fifty-fifty makeup may not always be the most favorable. As with soap molecules and proteins, the final superstructure that forms is guided by the relative sizes of the polar and hydrophobic sites or of the sites of different electric charge.
What Janus particles are good for
Anisotropic surface chemical makeup is interesting for applications even if one is not concerned with self-assembly. Think of optics, for example. If a particle is coated black on one side and white on the other, and if an electric field can make it flip, then you’ve got a switchable optical element that can be a pixel in an optical display. Switchable particles also enable the design of optical lenses whose shape and focal length adjust to environmental stimuli. Such tasks are especially easy to accomplish when the particles are immersed in fluid—hence the name of the field, liquid optics.
When Janus particles are designed properly, their anisotropic chemical makeup enables them to swim. The first example of self-propulsion came from catalytic reactions in which gas, evolved from a chemical reaction on one side of the particles, acted as a jet. Other applications will surely emerge once researchers can attach long chain molecules to one side of the particles. The chains will function like flagella.
Why not use Janus particles as soaps, “solid surfactants”? There are potential health reasons to do so, since some people are allergic to molecular soaps of the conventional kind. Solid surfactants should be especially effective in stabilizing mixtures of immiscible liquids. The reason is simple: Those clunky particles, which act like thousands of soap molecules glued together, adsorb strongly. For example, a mixture of oil and vinegar with Janus particles at the myriad interfaces would be unable to demix.
Even more exciting applications on the horizon will exploit Janus particles to form useful structures via a bottom-up approach of spontaneous self-assembly. Nature has long used that approach. For example, membranes form spontaneously from the assembly of phospholipids, a process that contrasts with the conventional top-down manufacturing approach with which cars, rockets, computer chips, and web-pages are fabricated. The bottom-up approach leads to more persistent and defect-free structures.
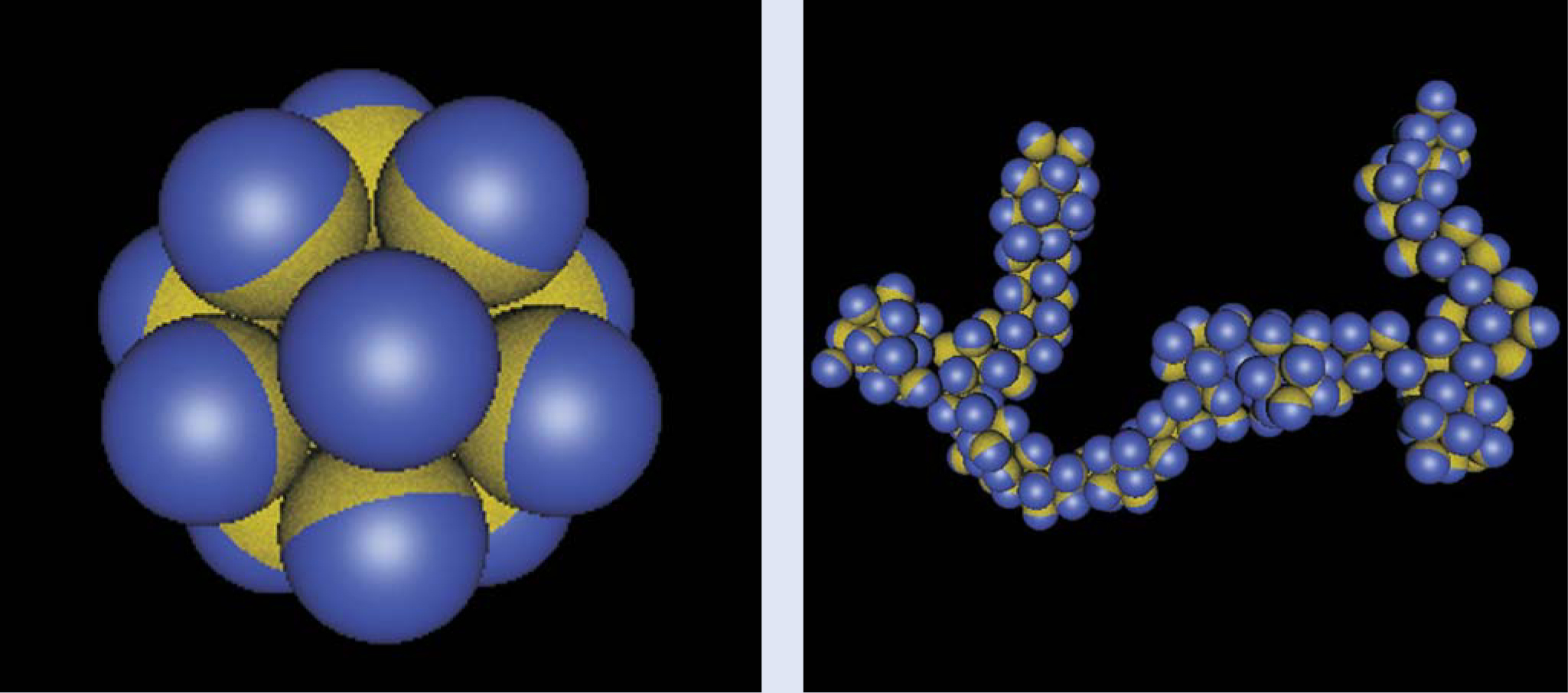
In that spirit, several research groups, including ours, have observed clusters of Janus particles forming spontaneously. Some resemble the micelles made by conventional soap molecules, but others are unexpectedly anisotropic in shape. Figure 2 shows examples that exhibit the beautiful symmetry of the micelle and the elegance of the elongated strings that form when individual micelles polymerize into superstructures. Such investigations may ultimately lead to responsive, adaptive structures. Smart new drug carriers are one example: At the sites of diseased tissues in the human body, mobile Janus particles can be triggered to form immobile clusters from which drugs are subsequently released.

Figure 2. Janus particles, hydrophobic on one side and polar on the other, assemble into larger structures when immersed in water containing dissolved salt. (a) This compact cluster of 12 particles is an example of a symmetric micelle structure. (b) Strings and branched anisotropic structures form when clusters join end to end. Here the hydrophobic side is half of the surface area; other structures form when one varies that fraction, the Janus balance.
(Images courtesy of Angelo Cacciuto and Erik Luijten.)
One of the most audacious long-term goals is to use particles to emulate how molecules fit together. Computer simulations show the promise of working with particles whose surfaces have more complex patch structures than just front and back—for example, four patches could emulate the tetravalent bonding of carbon atoms. Looking to the distant future, wouldn’t it be wonderful if one could reach into a soup of raw materials, trigger the appropriate Janus interactions, and pull out a self-assembled solar cell?
References
1. P.-G. de Gennes, Rev. Mod. Phys. 64, 645 (1992).https://doi.org/10.1103/RevModPhys.64.645
2. S. C. Glotzer, M. J. Solomon, Nat. Mater. 6, 557 (2007).https://doi.org/10.1038/nmat1949
3. L. Hong, A. Cacciuto, E. Luijten, S. Granick, Langmuir 24, 621 (2008).https://doi.org/10.1021/la7030818
4. S. Jiang, S. Granick, Langmuir 24, 2438 (2008).https://doi.org/10.1021/la703274a
More about the authors
Steve Granick is a professor of materials science and engineering, of chemistry, and of physics at the University of Illinois at Urbana-Champaign. Shan Jiang and Qian Chen are graduate students in the department of materials science and engineering at UIUC.
Steve Granick, University of Illinois, Urbana-Champaign, US.
Shan Jiang, UIUC.
Qian Chen, UIUC.




