When a novel metal alloy relieves stress, it chills out
DOI: 10.1063/PT.3.4288
The home air conditioner and refrigerator owe their success to vapor-compression technology. First, a compressor squeezes refrigerant vapor before it’s sent to a condenser. There the hot, pressurized gas cools and becomes a liquid. The liquid is then piped into an evaporator, where it depressurizes and sucks up the surrounding heat. As the environmental temperature decreases, the liquid gains heat and evaporates before reentering the compressor and repeating the process.
Although inexpensive, the vapor-compression refrigeration cycle is neither particularly efficient nor the most environmentally friendly process. Hydrofluorocarbons and other refrigerants inevitably leak out of the appliances and into the atmosphere, where they’re potent contributors to the greenhouse effect. Solid-state alternatives that apply a magnetic or electric field or stress to specially designed alloys have been gaining traction for decades. But the technology hasn’t quite achieved the temperature drop necessary to compete with commercially available cooling techniques (see the article by Ichiro Takeuchi and Karl Sandeman, Physics Today, December 2015, page 48
Nickel–titanium, nickel–manganese, and other shape-memory alloys have already been studied as potential solid-state refrigerants because they undergo structural phase transitions (see Physics Today, May 2010, page 20
Now Daoyong Cong of the University of Science and Technology Beijing and a team of engineers, materials scientists, and physicists have taken a different approach that focuses on the elastocaloric effect. Rather than optimize the magnetic properties of the material, the team adjusted the alloy composition with an eye toward maximizing the material’s volume change when squeezed. The elastocaloric effect generates a temperature change that could be big enough for large-scale refrigeration applications. 2
Designing a metallurgical marvel
The alloy Cong and his colleagues made, which comprises nickel, manganese, and titanium and is shown in figure
Figure 1.

A nickle, manganese, and titanium cylindrical sample (left) is loaded in a piston–cylinder pressure instrument. Applying stress from the top and bottom squeezes the sample, which decreases its volume and increases its temperature, the latter measured using a thermocouple (white ribbon). When the compressive stress is unloaded, the temperature reverses and the sample cools. That temperature drop makes it a promising material for solid-state refrigeration. (Photos by Shengwei Li.)

The first test alloy used a different composition of metals from previous studies: 50% nickel, 38% manganese, and 12% titanium. But differential scanning calorimetry measurements showed that the temperature at which the phase transition takes place is a bit too far above room temperature for an ideal refrigeration technology. After tweaking the Mn and Ti percentages in several more trials, the researchers settled on a composition, Ni50Mn31.5Ti18.5, that experiences a transition closer to room temperature. They also added a trace of boron to make the alloy less brittle.
Testing many different compositions was time well spent. Tuning the alloy’s Ti content changes its transition temperature, which makes the material useful for other heat pump applications. “I always thought that the best elastocaloric materials are not yet discovered, as nobody seriously investigated that possibility,” says Jaka Tušek of the University of Ljubljana in Slovenia. “Until now practically all the elastocaloric materials were simply taken from already known shape-memory alloys,” he says, “but this work shows that there is still significant room for improvement in developing new elastocaloric materials.” In addition, engineers still need to develop a refrigerator design that can repeatedly compress the material and efficiently expel waste heat.
In situ synchrotron high-energy x-ray diffraction experiments with the new alloy showed that the material’s volume decreases by 1.84–1.89% during its phase transition. The change is more than other similar shape-memory alloys as shown in figure
Figure 2.

Shape-memory alloys experience a structural phase transition when squeezed, which alters the metal compound’s crystal lattice. (a) The volume change and entropy change of a newly designed material (red circles) are larger than other materials with different metal stoichiometry. (b) Loading and unloading a stress of 700 MPa to a specially designed alloy comprising nickel, manganese, and titanium generated an adiabatic temperature swing of about 30 K. (Adapted from ref.
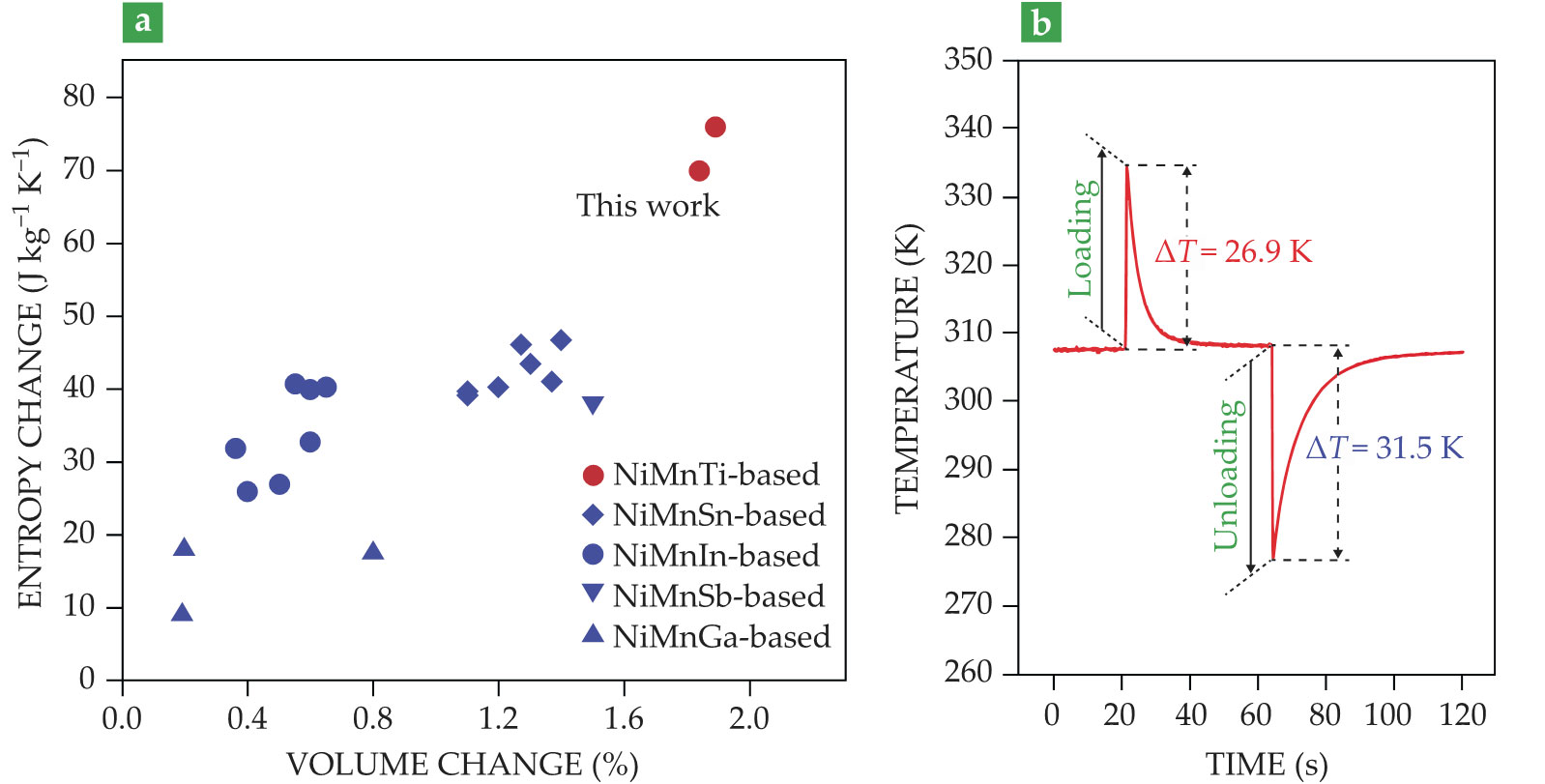
To test their hunch, the team applied 700 MPa of compressive stress to the sample and measured the temperature variation using a thermocouple as shown in figure 1. When compressed, the alloy experiences a temperature increase ΔT of about 30 K as shown in figure
Reaching a compromise
At least two big wrinkles still need to be ironed out. The material’s phase transition demands 700 MPa of stress, which is currently applied by a laboratory-scale instrument. A solid-state refrigerator will “need to effectively utilize the work released during the unloading of the material,” says Tušek, so that it can be affordable and efficient for commercial-scale applications. The material still needs to be tested to determine if it can withstand the millions of stress cycles a commercially available unit would need to perform. “Just as tweaking the composition led them to get this amazing ΔT, the fatigue is something that could be potentially engineered as well to get a material with a long life,” says Takeuchi.
However, altering an alloy’s composition has tradeoffs. Adjusting the structure to improve its strength or stability may decrease the magnitude of its elastocaloric effect. But, Takeuchi says, “They start off with such a high ΔT that it gives them room to do this adjustment.” So even though the elastocaloric effect may decrease if the material needs strengthening, the large temperature change already achieved affirms that it is a promising candidate for commercial solid-state refrigeration.
References
1. X. Moya, S. Kar-Narayan, N. D. Mathur, Nat. Mater. 13, 439 (2014). https://doi.org/10.1038/nmat3951
2. D. Cong et al., Phys. Rev. Lett. 122, 255703 (2019). https://doi.org/10.1103/PhysRevLett.122.255703
3. P. L. Potapov et al., Z. Metallkd. 87, 33 (1996);
S.-H. Chang, Mater. Lett. 65, 134 (2011); https://doi.org/10.1016/j.matlet.2010.09.073
Z. Y. Wei et al.., Appl. Phys. Lett. 107, 022406 (2015). https://doi.org/10.1063/1.49270584. G. L. Fraga, D. E. Brandão, J. G. Sereni, J. Magn. Magn. Mater. 102, 199 (1991). https://doi.org/10.1016/0304-8853(91)90287-K
More about the authors
Alex Lopatka, alopatka@aip.org





