Probing the Nanomechanics of Cartilage with Atomic Force Microscopy
DOI: 10.1063/1.1522202
Just a millimeter or so thick, the layers of cartilage in our knees can withstand compressive stresses of hundreds of kilopascals and tensile stresses ten times greater. Cartilage owes its remarkable resilience to the complex behavior and arrangement of its various molecular components. Unfortunately, like many carefully engineered devices, cartilage can go wrong. Twenty-one million Americans endure osteoarthritis, a painful degradation of cartilage in knees and other joints.
Candidate materials for synthetic cartilage are assembled from molecular building blocks. Drugs operate through molecular interactions. Treating cartilage diseases, therefore, depends on understanding the mechanical properties of cartilage at the molecular level. That goal is now one basic research step closer, thanks to a new technique for probing the nanomechanics of cartilage.
Seven years ago, MIT’s Alan Grodzinsky and Mike Buschmann, his graduate student at the time, identified the main source of cartilage’s compressive strength: electrostatic repulsion between glycosaminoglycan (GAG) molecules. 1 Now, Grodzinsky, his fellow MIT professor Christine Ortiz, and their students have used atomic force microscopy (AFM) to quantify, for the first time, the nanoscale forces exerted by GAG molecules. 2 Scaled up, the molecular forces match macroscopic measurements made on cartilage tissue.
Cartilage relies on three main molecules to bear loads: collagen, water, and proteoglycan. Mechanically, cartilage behaves like a water-logged bath sponge. Collagen, a protein, provides the springy scaffold and the tensile strength. Water offers significant resistance to compression, especially to high-frequency impulsive stress: The faster cartilage is squeezed, the harder it is to force water through the collagen scaffold and out into the synovial fluid that surrounds the cartilage. But the main source of cartilage’s compressive strength under equilibrium or low-frequency loading comes from the giant, negatively charged proteoglycan molecules that pervade the collagen scaffold in an aqueous gel.
The proteoglycans in cartilage are hierarchically structured combinations of proteins and sugars. At the lowest structural level are sugar molecules, which link together to form GAG molecules. The particular GAG molecules in cartilage, chondroitin sulfate GAG, are 30–40 nm long and consist mostly of about 20 sugar molecules, each of which features a negatively charged sulfate group.
GAGs are among nature’s most negatively charged molecules, but being charged isn’t enough to resist compression. The charges have to be held in such a way that the molecules respond to pressure by pushing against each other, rather than flying apart. That’s where the hierarchy comes in. After GAG, the next molecule in the structural hierarchy is the proteoglycan aggrecan (shown in figure 1). Each aggrecan consists of about a hundred GAGs attached 2–4 nm apart to a linear protein a few hundred nanometers long.

Aggrecan molecules, which are typically 450 nm long, form part of the cushioning basis of cartilage. The examples shown here were placed on a flat mica substrate for imaging with atomic force microscopy.
(Courtesy of Laurel Ng, MIT.)
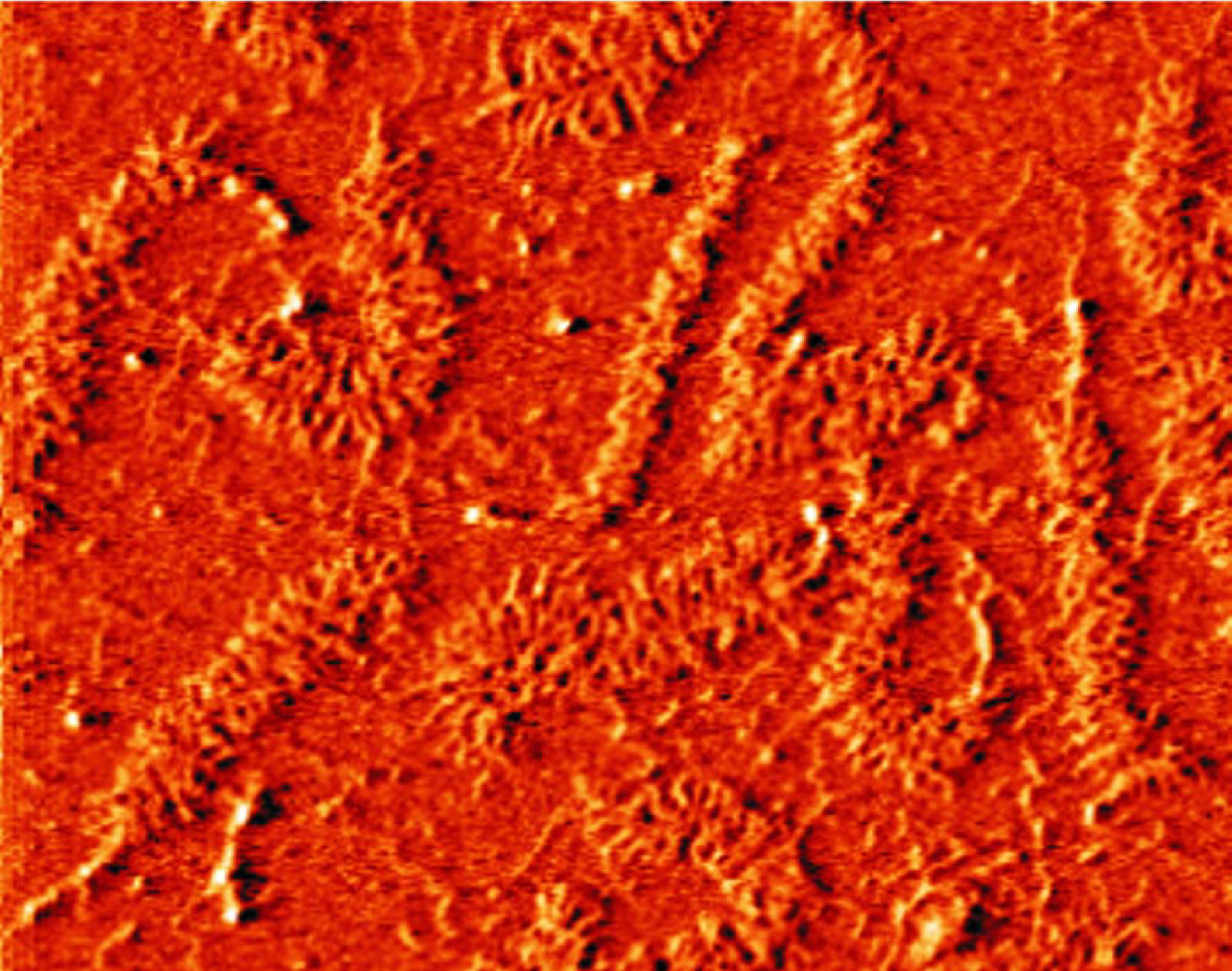
Aggrecans, in turn, form giant proteoglycan assemblies by attaching themselves to chains of hyaluronic acid. At about 10 μm long, aggrecan aggregates are so large that they can’t escape the collagen scaffold.
Prodding and probing
From a force point of view, the fundamental actors are the GAG molecules. Before prodding and probing GAGs with AFM, the MIT group first had to plant them on a substrate. Getting the GAGs to spontaneously stick their ends to a substrate with the right separation took three months of experimenting with various chemical conditions.
In the AFM experiments, which were performed by graduate student Joonil Seog, a piezoelectrically controlled apparatus drags a tiny cantilever tipped with an even tinier probe across the GAG-coated substrate in solution. The deflection of the cantilever—up for repulsion, down for attraction—is detected optically by bouncing a laser off the backside of the tip and recording the movement of the reflected beam. Hooke’s law, along with an independent measurement of the cantilever’s stiffness, provides the conversion of deflection to force.
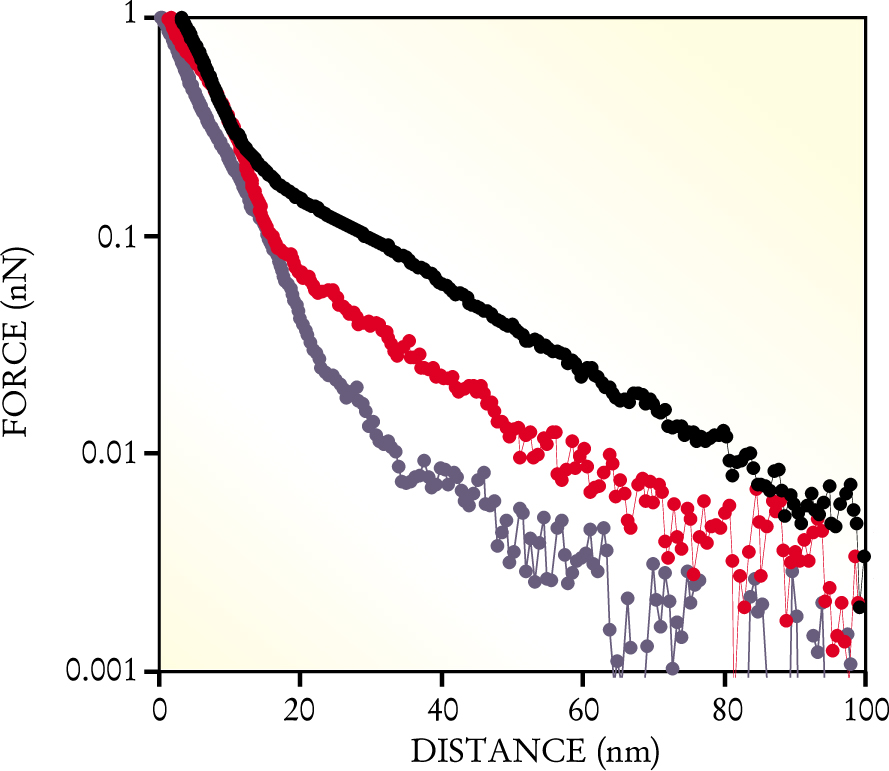
Such high-resolution force measurements are notoriously sensitive to the properties of the tip. To model and understand the experiment successfully, one has to know the tip’s geometry and mechanical properties and the distribution and density of charge on the tip. The MIT team took an incremental approach. First, they used a tip coated with electrically neutral and rather simple hydroxyl. Next, they used a tip coated with negatively charged sulfate groups. Finally, in the experiments that most resemble cartilage in vivo, they used a GAG-coated tip. Figure 2 shows the results of representative runs with the three tip coatings.
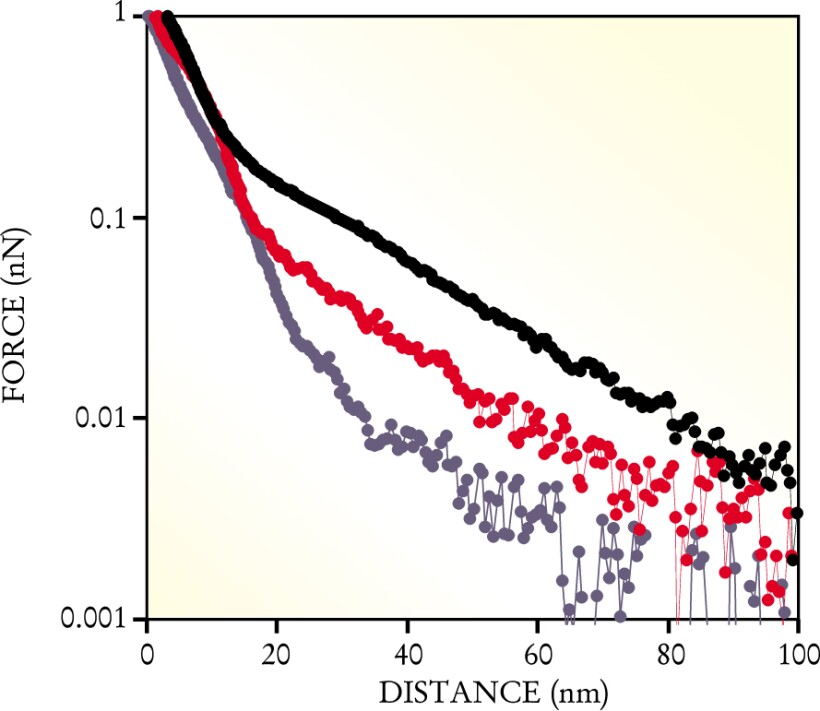
High-resolution measurements of the repulsive force between a GAG-coated substrate and a tip coated with various molecular groups: hydroxyl (blue), sulfate (red), and GAG (black). The GAG–GAG data capture one of the most important molecular interactions that occurs when cartilage is compressed.
(Courtesy of Joonil Seog, MIT.)
Although AFM measures total force, the mix of intermolecular forces is quite complex. The GAGs flop about in an aqueous solution that contains a mixture of ions. Neighboring and opposing GAGs repel each other electrostatically, but other forces are also present. Operating at shorter range are the van der Waals force and the hydration force, which comes into play when water is squeezed between the GAGs. Steric forces, which are typically longer-ranged than the van der Waals and hydration forces, resist attempts to squash the long GAG molecules.
To resolve the forces’ individual contributions, the MIT group took advantage of the forces’ different dependences on the salinity and acidity of the surrounding solution. The electrostatic force, for example, weakens when the concentration of cations increases. Seog, therefore, ran the experiments at different values of salinity and acidity that spanned a wide range above and below physiological conditions.
Another of Grodzinsky and Ortiz’s students, Delphine Dean, modeled the various forces and their dependences. One of her models, an application of the mean-field Poisson–Boltzmann theory of electrolytes, matched the data fairly well and strongly suggested the predominant role of electrostatic forces. In particular, her modeling suggests that the GAGs attached to the tip and the GAGs attached to the substrate interpenetrate as they’re compressed, increase the local charge density, and, as figure 2 shows, boost the repulsive force.
Implications
For all its wonderful mechanical properties, cartilage has a serious drawback: It lacks a blood supply. Nutrients must somehow diffuse into the tissue to reach the few cells that can manufacture new molecules. As a result, tissue repair is ineffective, and, once damaged, cartilage keeps wearing away, eventually to the bone. Joint replacement is often the only relief from the pain.
Can the AFM studies lead to a treatment? Maybe. Anna Plaas, who, with Shirley Wong-Palms, supplies the MIT group with GAG molecules, has been chemically analyzing GAGs from diseased cartilage in her lab at the University of South Florida. Compared with healthy ones, diseased GAGs turn out to be shorter and have less charge. By probing diseased GAGs, the MIT group hopes to discover what chemical defects produce what mechanical defects. Drugs, which operate in the chemical arena, might then be developed to buttress weakened GAGs.
References
1. M. D. Buschmann, A. J. Grodzinsky, J. Biomech. Eng. 117, 179 (1995).https://doi.org/10.1115/1.2796000
2. J. Seog, D. Dean, A. H. K. Plaas, S. Wong-Palms, A. J. Grodzinsky, C. Ortiz, Macromolecules 35, 5601 (2002).https://doi.org/10.1021/ma0121621




