New kind of ultralong dimers are observed
DOI: 10.1063/1.3141927
In the early 1930s, Enrico Fermi set himself the task of explaining some puzzling spectroscopic observations. 1 The absorption lines of highly excited alkali atoms, when buffered by inert gas, didn’t broaden as expected but shifted in wavelength. Further confounding expectations, the direction of the shift depended on the species.
Fermi could account for the shifting lines by postulating that the loosely held electron of a highly excited alkali atom interacts with a buffer atom only at near-zero range. Half a century later, groups led by Françoise Masnou-Seeuws, Eugen de Prunelé, and Chris Greene showed that Fermi’s interaction produces a long-range oscillatory potential between the atoms. However, for the light atoms the theorists studied and for the conditions experimenters could reach, the potential’s valleys seemed too shallow to bind the two types of atom in dimers.
By the late 1990s, physicists could trap and cool atoms to the nanokelvin temperatures needed to form Bose–Einstein condensates. Inspired by that feat, Greene, Alan Dickinson, and Hossein Sadeghpour resurrected the oscillatory potential and proposed in 2000 that it could bind dimers at or near BEC temperatures and densities. 2 Because of the potential’s intrinsic weakness, the putative dimers would be tens, even hundreds, of nanometers across.
Now, Tilman Pfau of the University of Stuttgart in Germany and his collaborators have observed the novel binding mechanism in the case that Greene, Dickinson, and Sadeghpour identified as the most promising: dimers that consist of a rubidium atom in its ground state and a rubidium atom in a highly excited Rydberg state. 3
The Rb dimers that Pfau’s group made add a fifth class of chemical bond to the familiar set of ionic, covalent, hydrogen, and van der Waals. The dimers are big indeed. At 80 nm, they’re wider than a ribosome, the 5-megadalton macromolecule that translates our DNA into protein, and longer than the transistor gates in the newest, most powerful microprocessors.
Rydberg atoms
Rydberg atoms—that is, atoms excited to states of high principal quantum number n—interest experimenters in part because the atoms can be manipulated with high precision using microwaves and narrow-band lasers. That property makes Rydberg atoms a convenient testbed for studying the nature of quantum mechanics and its applications. (See Physics Today, June 2007, page 21
To create a sample of dense, cold Rb Rydberg atoms, one typically starts at temperatures around 100 µK and densities between 1010 and 1011 atom cm-3. To make a BEC, one needs to reach temperatures 1/100th as low and densities 1000 times as high. Five years ago Pfau started exploring that colder, denser regime to look for new quantum phenomena. Collective coherence, which could lead to applications in quantum computation, was one broad goal. Ultracold chemistry, including the dimers that Greene, Dickinson, and Sadeghpour had predicted, was the other.
Rubidium was a natural choice for dimerization. As the first species coaxed into a BEC, Rb’s cooling transitions are well known. Moreover, as Greene and company had noted, the atom’s relatively high mass deepens the dimer potential.
In their 2000 paper, the three theorists provided a script for making the dimers in the lab. The role of polarizable species is played by ground-state Rb. The role of highly excited partner is played by Rb atoms with n in the 20–80 range and angular momentum quantum number l in the 0–20 range.
On paper at least, all combinations of n and l yield dimers. For practical reasons Pfau chose to pair the ground state with Rydberg states of n around 35 and l equal to zero. Figure 1 shows the calculated probability density of the outermost Rydberg electron for that combination of n and l, the binding potential of the Rydberg electron and the ground-state atom, and two bound-state wavefunctions.

Figure 1. The calculated probability density of an electron in the Rydberg orbit of a rubidium atom resembles a corrugated disk (purple and yellow surface) when the electron is adjacent to a polarizable atom, in this case Rb in its ground state. The interaction between the Rydberg electron and the ground-state atom establishes an interatomic potential (green line) that creates two bound vibrational states (blue shading) in the potential’s outermost valley.
(Adapted from
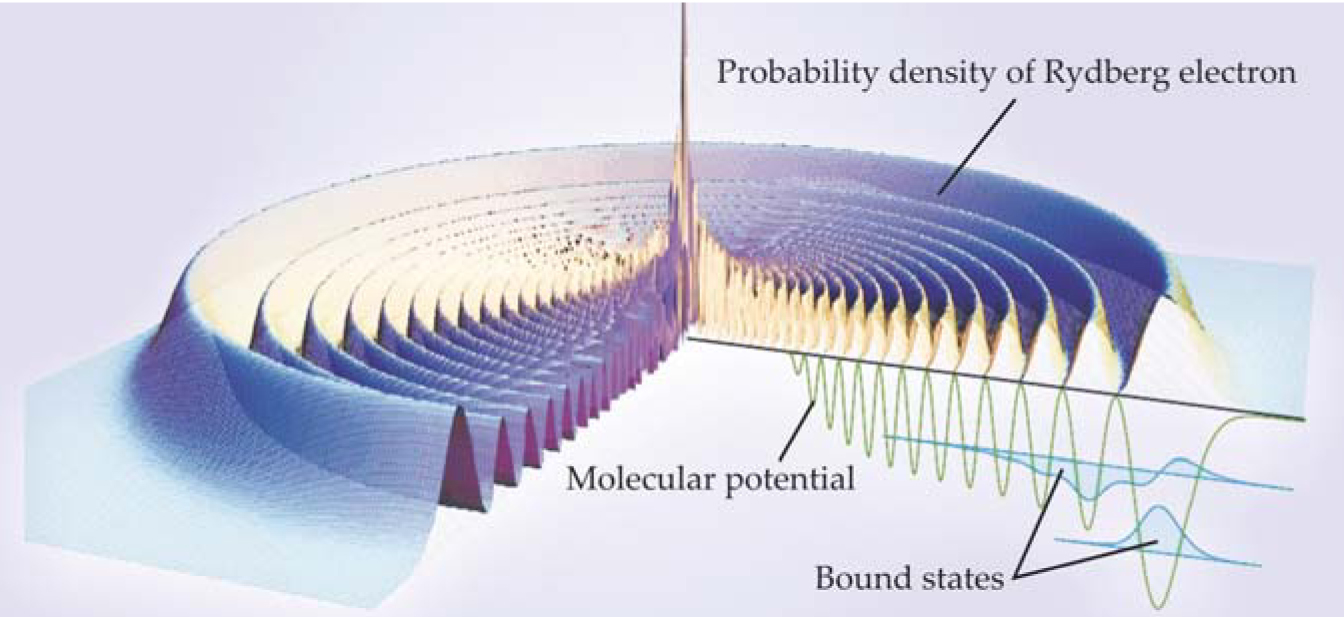
The wavy nature of the dimer’s potential comes from the Rydberg electron. If, as Fermi first noted, the ground-state atom’s scattering length is negative, the potential develops bond-forming minima.
Three of Pfau’s graduate students, Vera Bendkowsky, Björn Butscher, and Johannes Nipper, set up and ran the experiment to observe the dimers, along with Robert Löw. James Shafer, a visitor from the University of Oklahoma, helped interpret the results. It begins by cooling Rb atoms to just above the conditions for Bose–Einstein condensation. Next, two-photon excitation kicks a fraction of the atoms up to a Rydberg state of predetermined n and l.
After a few-microsecond burst of laser excitation, the gas, which contains a mix of ground-state atoms, single Rydberg atoms, or dimers, is ionized by an adjustable electric field. The number of ionized atoms or dimers detected at each value of the field manifests the binding-energy spectrum. Figure 2 shows three examples at different values of n.

Figure 2. The existence of dimers formed by Rydberg and ground-state atoms is determined by counting the number of atoms ionized by an adjustable electric field. At the center of each plot is a pair of unequal peaks that arises from single, unbound Rydberg atoms; for easy comparison, the peak position (measured in frequency units) is set to zero. Dimers in the vibrational ground state (v = 0) appear in each spectrum as lower-energy peaks. Peaks in the first vibrational state (v = 1) also appear, as do peaks related to additional scattering processes.
(Adapted from
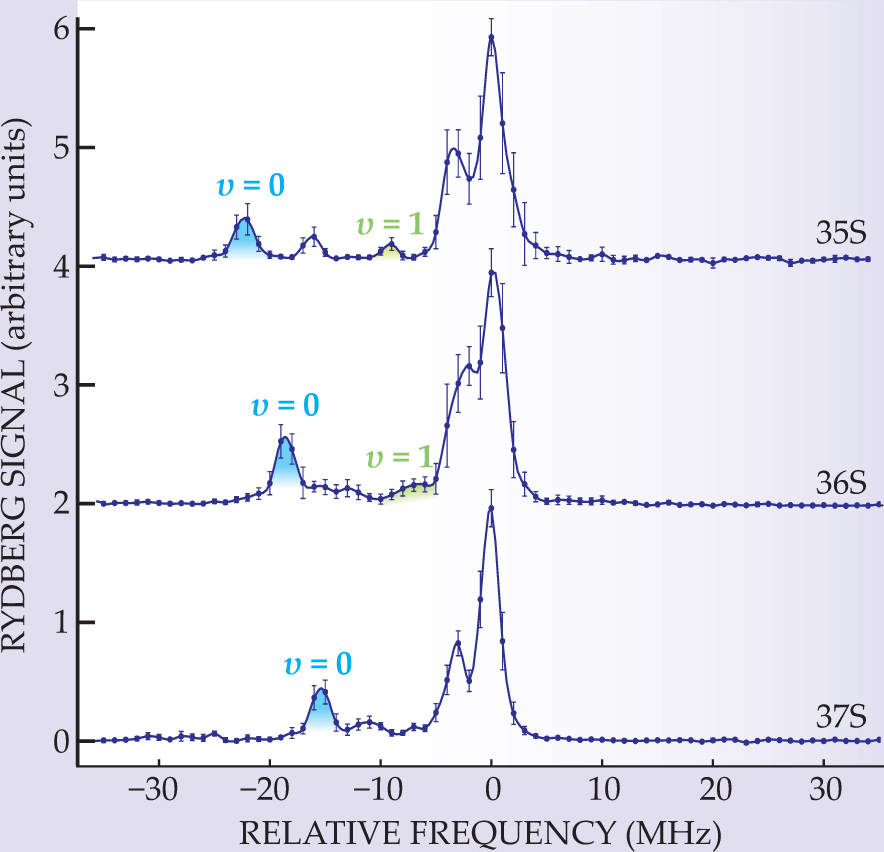
According to Greene and company’s theory, the most stable dimers are in the ground and first excited state of the dimer’s vibrational potential. Having observed peaks for values of n from 34 to 40, the Stuttgart team had enough dynamic range to test those and other predictions.
Only one adjustable parameter, Rb’s s–wave scattering length, features in the model the Stuttgart group built from Greene and company’s theory and its subsequent refinements. Allowing the scattering length to vary yields perfect fits to the two curves of n versus binding energy: one for the vibrational ground state, the other for the first excited vibrational state. Presuming the model to be correct yields a value of the scattering length of −18 Bohr radii, which is consistent with ab initio calculations.
The experiment brought some surprises. At 15–18 µs, the lifetime of the dimers is one-third as long as the lifetime of the Rydberg atoms. There’s no quantitative explanation for that unexpected result. Qualitatively, inelastic scattering of the Rydberg electron from the ground-state atom could cause the decay. Figure 2 shows another surprise: peaks in the spectra between those due to the dimer’s vibrational states. Higher-order partial-wave scattering appears to be the likely culprit.
The vibrational l = 0 dimers that the Stuttgart group observed represent the first in a potentially rich landscape of dimer species. After installing additional equipment, Pfau says he can reach much larger values of l. Based on the appearance of their electron probability densities, Greene has dubbed those states trilobites and butterflies.
References
1. E. Fermi, Nuovo Cimento 11, 157 (1934). https://doi.org/10.1007/BF02959829
2. C. H. Greene, A. S. Dickinson, H. R. Sadeghpour, Phys. Rev. Lett. 85, 2458 (2000). https://doi.org/10.1103/PhysRevLett.85.2458
3. V. Bendkowsky et al., Nature 458, 1005 (2009). https://doi.org/10.1038/nature07945




