Metal–organic framework extracts water from thin air
DOI: 10.1063/PT.3.3579
An average-sized living room of 90 m3 in a temperate climate—25 °C and 60% relative humidity—contains about a kilogram of water vapor dispersed in the air. Even in the desert, where liquid water is scarce, relative humidity typically hovers around 20%, so there’s still plenty of water in the air. Because it’s constantly refreshed by evaporation from the ocean and atmospheric circulation, the water in the atmosphere can be considered a limitless renewable resource.
Now Omar Yaghi of the University of California, Berkeley, Evelyn Wang of MIT, and their colleagues have taken a step toward tapping that resource by creating a water-collecting device that works at relative humidity as low as 20% and requires no external energy source. 1
Their concept is based on a metal–organic framework (MOF), a highly porous material whose properties can be tuned by varying the metal and organic components and how they’re put together. With their MOF-based devices, such as the one in figure

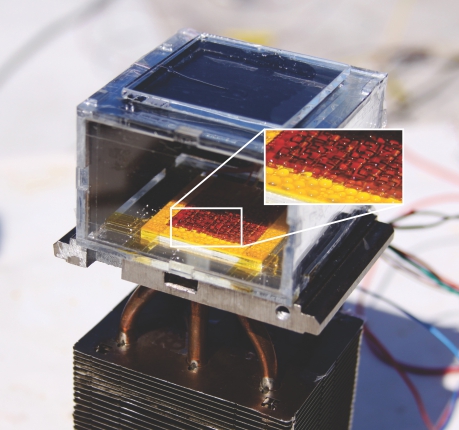
Figure 1. A water harvester on an MIT rooftop consists of a small sealable chamber containing a temperature-controlled condenser (the yellow and orange square, 5 cm on a side) and a layer of humidity-attracting metal–organic framework (not visible). As shown in the inset, drops of water collect on the condenser even though its temperature is the same as that of the outside air. (Courtesy of Evelyn Wang.)
Any drop to drink
Hot air can hold many times as much water vapor as cold air can. When a volume of air holding a given amount of moisture is cooled, therefore, there comes a temperature—the dew point—at which the water has no choice but to condense. The phenomenon is exemplified by the dewdrops that cling to blades of grass on a cool morning—or by the fogging of eyeglasses when one enters a warm room.
Schemes for harvesting the water that nature obligingly condenses into dew are nothing new. 2 In arid regions, however, the daytime relative humidity may be so low that the nighttime temperature never drops below the dew point.
Happily, many so-called hygroscopic materials exist that, by virtue of their surface or bulk properties, can extract water from the air even above the dew point. Sugar is a familiar example: Dry hard candies become sticky when exposed to humid air for too long. Another example is silica gel, a porous form of silicon dioxide commonly used as a desiccant. Because silica gel has both a strong surface affinity for water molecules and a high surface area, it can hold up to 40% of its own weight in water.
The water held by a hygroscopic material is not yet in liquid form, but it can be made so; designs for desiccant-based water harvesting systems 2 have been proposed and patented since at least the 1940s. Their basic operating principle is as follows: Cool, moist air circulates over the desiccant, usually at night. When the desiccant has captured enough moisture, it’s sealed in a small space and heated to force the water to re-evaporate. The moisture-laden air is cooled to the ambient temperature to condense the water, and the desiccant is ready to be used again.
The problem is that conventional desiccants stop working at low relative humidity, require an impractically high regeneration temperature, or both. For example, silica gel no longer takes in water at around 40% humidity, and it must be heated to around 120 °C to extract the water it’s holding. Other conventional materials can improve on one of those limitations or the other, but not both at the same time. The dilemma can be understood as a simple trade-off: Materials with lower affinity for water have higher cutoff humidities, and those with higher affinity for water must be heated to higher temperatures to get that water out.
MOF-801
A growing class of designer porous materials, MOFs are built out of metal-based nodes and carbon-based links arrayed in a regular crystalline structure. The first stable MOFs were reported in 1999 by two groups, including Yaghi’s. 3 Tens of thousands of MOFs are now known, and they’re being explored for applications such as catalysis and gas storage. 4 Their appeal stems from their customizability: By choosing the right metal and organic units, one can independently tune the pores’ size, shape, topology, and surface chemistry.
The new water harvester uses a material called MOF-801; shown in figure

Figure 2. The metal–organic framework MOF-801 (a) is made of zirconium oxide clusters (blue polyhedra) and fumaric acid molecules (whose atoms are indicated by black and red dots). The size, shape, and surface chemistry of its internal pores (yellow, orange, and green) make it an ideal water collector. Because its isothermal water-uptake curves (b) feature a sharp upswing at low relative humidity, it can capture water even in dry air and release it under modest heating. (Adapted from refs.
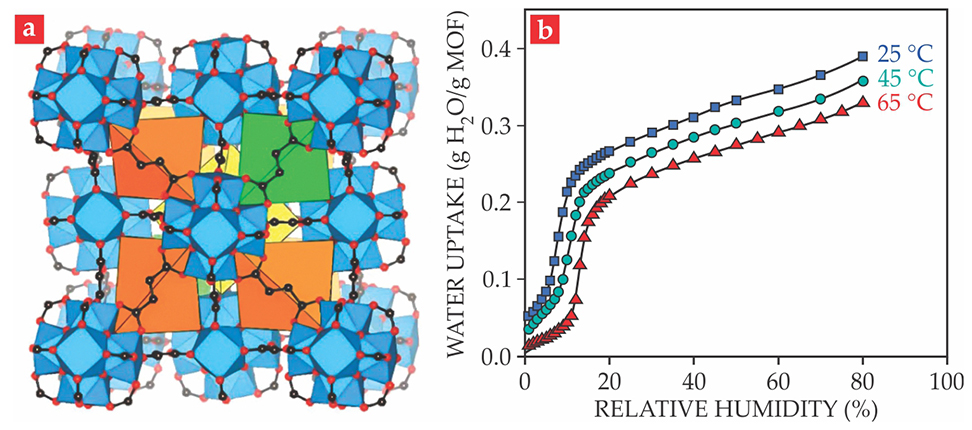
X-ray and neutron diffraction of water-saturated MOF-801 helped reveal how the material can overcome the tradeoff between high regeneration temperature and high operation humidity. The MOF’s interconnected pores have structurally distinct shapes: tetrahedral (orange and green in figure
That strong network of interactions among stored molecules, with fairly few bonds between the water and the MOF, is presumed to be why MOF-801 takes in water so readily but also releases it easily. Furthermore, Yaghi and colleagues found that the material can endure many adsorption–desorption cycles with no structural damage.
Heat and water
Having created the new MOF, Yaghi struck up a collaboration with Wang, an engineer with an interest in thermal systems, to make use of it. At first, the application they had in mind was a so-called thermal battery. When water (or anything else) is adsorbed onto a surface, heat is released; conversely, when water returns to the gas phase, heat is consumed. A device that can harness that heat of adsorption and desorption, using water from a self-contained reservoir, could be a more efficient means of climate control in electric cars than running an electric heater or air conditioner off the car battery. 6 The water-harvesting idea grew out of the thermal battery project. “It operates based on the same physics,” says Wang.
The proof-of-principle water collector, designed and built by Wang’s student Hyunho Kim, is conceptually similar to other desiccant-based systems. Air is passed over the MOF in an open chamber to saturate it with water. Then the chamber is closed and the MOF is heated. Water is released and quickly liquefies on an ambient-temperature condenser.
Importantly, though, the 65 °C needed to regenerate MOF-801 is much easier to achieve than the 120 °C required to extract the water from silica gel. Direct but unconcentrated sunlight can heat a dark-colored surface to a considerably higher temperature than the ambient air. On a 25 °C day, such simple solar heating can easily reach 65 °C.
Wang and colleagues used a heating panel darkened with graphite, which can be seen on the top of the demonstration chamber in figure
The condenser at the bottom of the chamber was held at the ambient temperature even as the graphite and MOF were heated by the Sun. Sure enough, drops of water—visible in the inset—began to accumulate. For simplicity in their demonstration, the researchers chose to use active refrigeration to maintain the condenser’s temperature. But for off-the-grid operation, they could just as easily have cooled it passively by connecting it to a heat sink.
Because the demonstration used less than 2 g of MOF, the collected water amounted to just a fraction of a milliliter. Wang and colleagues estimate that a larger device could harvest more than 200 mL of water per kilogram of MOF-801 under 20% relative humidity. Furthermore, if the MOF layer were made sufficiently thin, it could adsorb and desorb water quickly enough to perform up to 12 heating and cooling cycles per day. (Cooling the MOF from its 65 °C high is as simple as shading the graphite panel from sunlight.) In that case, a device could produce up to 2.8 L of water per kilogram of MOF per day.
Dust and other airborne particles can condense along with the water vapor. “We expect a filter would be needed, as it is with other atmospheric water generators,” says Wang. “But we don’t anticipate any other issues with the cleanliness of the water.”
What may be an issue, however, is cost. Although zirconium, the metal component of MOF-801, is not among the rarest elements, it costs around $150 per kilogram. Yaghi and his group are working on replicating MOF-801’s properties with cheaper metals such as aluminum. Still, says Wang, “We don’t anticipate that this technology will ever compete with other nontraditional water systems such as desalination. It’s more suitable for isolated regions where there’s no water infrastructure, or no water at all.”
References
1. H. Kim et al., Science 356, 430 (2017). https://doi.org/10.1126/science.aam8743
2. B. Khalil et al., Sustain. Water Resour. Manage. 2, 71 (2016) and references therein. https://doi.org/10.1007/s40899-015-0038-z
3. S. S.-Y. Chui et al., Science 283, 1148 (1999);https://doi.org/10.1126/science.283.5405.1148
H. Li et al., Nature 402, 276 (1999). https://doi.org/10.1038/462484. H. Furukawa et al., Science 341, 1230444 (2013). https://doi.org/10.1126/science.1230444
5. H. Furukawa et al., J. Am. Chem. Soc. 136, 4369 (2014). https://doi.org/10.1021/ja500330a
6. S. Narayanan et al., Appl. Energy 149, 104 (2015). https://doi.org/10.1016/j.apenergy.2015.03.101
More about the authors
Johanna L. Miller, jmiller@aip.org




