Making molecular-spin qubits more robust
DOI: 10.1063/PT.3.3157
The allure of a quantum computer has drawn many researchers to study a wide variety of two-level quantum systems to represent quantum bits of information. Depending on the state such a “qubit” occupies, it might represent a binary “0” or “1,” or a superposition of those states. Physical realizations of qubits developed so far include the flux in a superconducting system, the polarization of a photon, and the electronic or nuclear spin of an atom or ion. Each realization has its own set of strengths and weaknesses.
One other candidate is the electron spin carried by a molecule, especially a molecule with one or more metal ions. Such molecular nanomagnets, which can have large spins, are embedded in a solid-state system such as a crystal. The attraction of molecular systems arises from the ability of chemists to synthesize a large number of identical entities and to tailor the magnetic properties of those entities. Chemists have acquired a strong expertise in tuning, controlling, and manipulating the properties of molecules and hence can create tunable devices, such as molecular-spin transistors or spin valves, with new capabilities.
Early hopes for molecular magnets have been frustrated, however, by decoherence—the destruction of the qubits’ information caused by interactions with the environment. In spin qubits, much of the decoherence comes from dipolar interactions with other electron spins; the rest stems from interactions with phonons and with the surrounding nuclear spins. Until now, decoherence in nanomagnets could be significantly mitigated only by polarizing the electronic spins in a large magnetic field or by greatly diluting the concentration of spins. Neither of those conditions is propitious for practical qubit operation: the former because of the difficulty of operating at a high field and the latter because of the scarcity of near-enough neighbors for desired qubit entanglement.
There is now a third way to reduce decoherence in molecular magnets, thanks to the work of a team led by Stephen Hill at the National High Magnetic Field Laboratory and Florida State University and by Eugenio Coronado at the University of Valencia. 1 They used an approach known as the “clock transition,” first introduced 31 years ago to make atomic clocks based on trapped ions immune to magnetic field variations and other environmental perturbations. Such immunity is desirable for spin qubits as well because decoherence sources can manifest themselves partly as magnetic field fluctuations. Indeed, the clock transition approach has been used to lengthen coherence times in several other qubit systems, including another solid-state, electron-spin qubit candidate: bismuth donors in silicon. 2 Coherence times for low concentrations of the bismuth-spin system were on the order of seconds.
The idea behind the clock transition approach is to operate the system at a sweet spot where the energy splitting between a qubit’s 0 and 1 states is insensitive to environmentally caused variations in the magnetic field. Not every electron-spin system has such an operating point, but Hill, Coronado, and their colleagues recognized that one such system did: the holmium-containing molecular nanomagnet shown in figure 1a. Working with the crystals of that molecule set at a clock transition, they measured coherence times as long as 8 μs. That’s comparable to coherence times previously measured in molecular-spin samples that were about one hundred times more dilute. Although still shorter than desirable, 8 μs is long compared to single-qubit operation times, which are on the order of tens of nanoseconds.

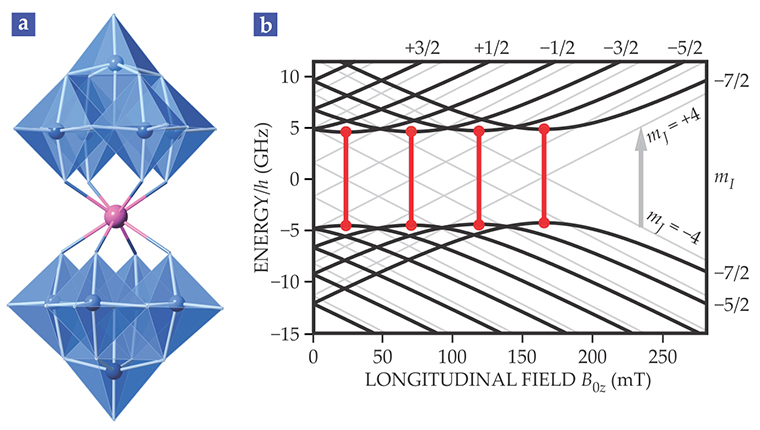
Figure 1. Energy level diagram for a holmium molecular nanomagnet. (a) The magnetic holmium molecule consists of a holmium ion (purple) sandwiched between two complexes of tungsten atoms (blue) and oxygen atoms (at the vertices of the polyhedra). (b) The hyperfine energy levels in a fully symmetric molecule (light gray lines) are shifted and bent (black lines) because the molecule has a slight axial asymmetry. Transitions that connect the extrema of the curves (red lines) are the clock transitions: The frequency of such transitions is insensitive to fluctuations in the magnetic field. (Adapted from ref.
Recipe for a clock transition
To realize a sweet spot for avoiding decoherence, one needs a system whose energy-level diagram features a large gap between different hyperfine spin levels, so that those levels do not cross. Within that gap, there should be points—corresponding to the minimum and maximum of the two levels—where the frequency f of the signal for transitioning between two levels is independent of the magnetic field B. That is, to first order, df/dB = 0. That requirement determines the position of the clock transition.
Consider the molecule pictured in figure 1a. The holmium ion (purple) is trapped between two molecular moieties of tungsten (blue) and oxygen atoms (at the vertices of the polyhedra). The two ligands create a square antiprismatic cage around the holmium with an approximate fourfold symmetry. Minor deviations from that axial symmetry generate the series of anti-crossings between levels in which clock transitions can occur.
The hyperfine spin levels for the holmium molecule are shown as a function of applied magnetic field in figure 1b. If the molecule were perfectly symmetrical, its hyperfine spin levels, shown as pale gray lines, would cross. Because of the axial asymmetry the hyperfine levels, shown in black, curve away from each other, opening a gap. The clock transitions, shown in red, occur at extrema in the energy-level curves.
Hill, Coronado, and their team measured the coherence time for the molecular qubits by using pulsed electron paramagnetic resonance to detect a spin echo. If the spins are coherent, says Hill, they “talk back”; the characteristic time for decay of the echo signal is a measure of the coherence time.
The Florida–Valencia group grew crystals of the holmium-containing molecule. To vary the spin concentration, the researchers substituted nonmagnetic yttrium ions at the sites of the holmium ions. They then measured the coherence times for four different densities of spins, ranging from 0.1%, or one magnetic molecule for every 1000 matrix molecules, to 25%. (Dilute systems have molecular-spin densities of about 0.01%.)
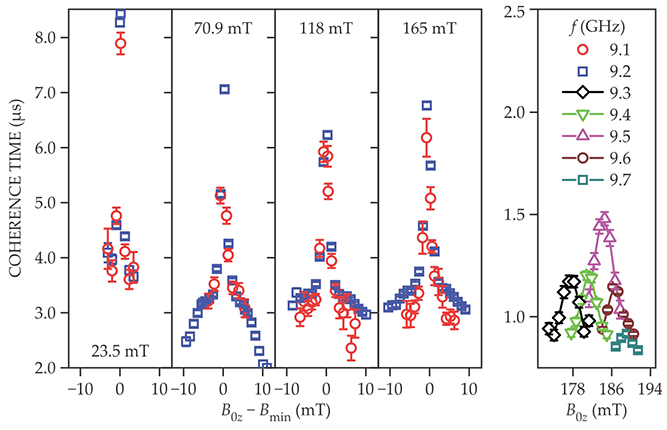
The coherence times for the low end of the concentration range, measured by setting the magnetic field at four different clock transitions, are shown in figure 2. The values peak near 8.4 μs. Coherence times still reach about 8 μs at concentrations of 1% and fall to about 0.7 µs at 10%. At 25%, the spin echo can no longer be detected. Away from the clock transition points, the spin coherence times are less than 1.5 µs at 0.1% concentration, as shown in the rightmost panel.

Figure 2. Coherence times. The first four panels indicate coherence times at four different clock transitions. Red data points are measured at a frequency of 9.1 GHz; blue data points, at 9.2 GHz. The magnetic fields at which those clock transitions occur are given in each panel, and the horizontal axis plots deviations from each value. The rightmost panel shows that coherence times are shorter when measured at various frequencies away from the clock transitions. (Adapted from ref.
The new results show that it’s possible to fortify systems of molecular spin against decoherence at high concentrations. Coronado points out that the same principles that govern the existence of a clock transition in holmium-containing molecules can be applied to the design of other robust spin qubits. His team already has some candidates.
Experimental studies of decoherence have been guided by theoretical studies of interactions in a dissipative environment. Philip Stamp, a theorist at the University of British Columbia in Canada, whose work on decoherence mechanisms helped lead to the prediction and observation of dipolar decoherence, 3 , 4 stresses that dipolar interactions are particularly dangerous because, unlike most other decoherence mechanisms, they are long range: They entangle a given qubit with distant spins, not just with selected nearby neighbors. Further understanding of such dipolar interactions will be enormously important as all kinds of qubit systems scale up to larger ensembles.
Conflicting roles of interaction
As candidates for construction of a quantum computer, molecular spins still face many hurdles. Chief among them is the tension that exists in all quantum computing systems: You need to avoid coherence-destroying interactions with other qubits, and yet you need communication with those qubits to perform the basic operations of the computer. Can one get rid of the dipolar interactions that cause decoherence and still allow qubits to selectively interact with their neighbors? One possibility is to set the magnetic field away from the clock transition while engineering strong interactions between qubits and then return the system to the clock transition to preserve the quantum state between operations.
Another possibility is to exploit the stability of some molecular magnets. They can be plucked from the crystals in which they sit and placed elsewhere, such as on a surface, in a structure that couples two or more such qubits. The challenge would be to couple the qubits controllably while avoiding unwanted interactions. As an example of one step in that direction, a team led by Wolfgang Wernsdorfer of the Néel Institute in Grenoble, France, has coupled a single-molecule magnet with a carbon nanotube to study electrically driven nuclear-spin resonances. 5
Andrea Morello of the University of New South Wales in Australia points out that, although molecular spins might currently be less developed than other platforms for quantum computing, they have nevertheless demonstrated a potential to serve as a testing ground for basic ideas and techniques relevant to quantum information. “The current surge of research on functionalized molecules on surfaces also offers interesting avenues to engineer patterns and interactions between the molecules.” Morello started his career in that “fantastic school of physics,” and says it has given him a significant head start for working on spin-based quantum systems.
Wernsdorfer echoes Morello’s sentiments: “These devices are exciting experimental playgrounds for learning about electronic transport through a single molecule, discovering new mesoscopic phenomena, and testing quantum effects at the single-molecule level.”
References
1. M. Shiddiq et al., Nature 531, 348 (2016). https://doi.org/10.1038/nature16984
2. G. Wolfowicz et al., Nat. Nanotechol. 8, 561 (2013). https://doi.org/10.1038/nnano.2013.117
3. A. Morello, P. C. E. Stamp, I. S. Tupitsyn, Phys. Rev. Lett. 97, 207206 (2006). https://doi.org/10.1103/PhysRevLett.97.207206
4. S. Takahashi et al., Nature 476, 76 (2011). https://doi.org/10.1038/nature10314
5. Stefan Thiele et al., Science 344, 1135 (2014).https://doi.org/10.1126/science.1249802




