Laser pulses probe quantum beats
DOI: 10.1063/PT.3.4957
Cryptochromes are a class of light-sensitive proteins found in many organisms. In animals, they’re an integral part of the circadian clock: the collection of biochemical oscillations that align physiology with the day–night cycle. 1 A network of positive and negative feedback loops in gene expression and protein production couple those oscillations to downstream processes. For example, they link external light and temperature conditions to levels of hormones that stimulate hunger and sleepiness.
The involvement of cryptochromes in circadian clocks is well established; they’re part of a negative feedback loop that suppresses transcription. But much about them remains poorly understood—for example, their structures are not well characterized, and the light-reactive proteins participate in cycles that don’t have light as an input.
Cryptochromes are also the only biomolecules other than chlorophylls that are known to host so-called radical pairs. When a photon hits a cryptochrome, it can excite an electron that hops along the molecule from an electron donor to an electron acceptor—areas with low and high electron affinities, respectively. The mobile electron leaves behind another electron, but the electrons’ spins remain entangled despite the separation.
The paired electrons exist in a singlet state before the excitation because they must have opposite spins per the Pauli exclusion principle. And they usually remain that way afterward. But when conditions are just right, the electrons undergo quantum beats: oscillations between singlet and triplet states. The frequency of the beats is determined by the electron spins’ magnetic environment, which is dominated by the magnetic nuclei in the molecule, and the electrons’ state can affect which reaction pathways are available to the molecule.
Unfortunately, the beats are tricky to study: The states’ nearly equal energies make them indistinguishable by optical spectroscopy techniques. But Christoph Lambert and his graduate students David Mims and Jonathan Herpich (University of Würzburg, Germany), in collaboration with theoreticians Ulrich Steiner (University of Konstanz, Germany) and Nikita Lukzen (Novosibirsk State University, Russia), have devised a work-around. Rather than measuring the states’ energies, the optical method measures the singlet and triplet populations through their decay products. 2
Unlike the existing spin resonance technique for measuring quantum beats, the optical approach isn’t limited to certain magnetic field conditions. It also has the potential to extend the study of spin chemistry by boosting temporal resolution by up to two orders of magnitude.
Pump–push pulses
“Years ago, I encouraged David Mims to set up the pump–push experiment to see whether we could shift electrons back and forth in molecular dyads and triads using two laser pulses,” says Lambert. But before Mims could do so, he needed the right experimental subject. In 2019, Mims and Herpich designed and synthesized a molecule with electron donor and acceptor moieties that were familiar to the Lambert group.
For the system to form a charge-separated state (CSS) that the researchers could study, the donor and acceptor had to be connected in just the right way. The link they inserted, tetramethyl dihydroanthracene, provides a weak electronic coupling between the two electrons. It holds them about 20 Å apart so they remain in a CSS for at least 100 ns—long enough to observe beats. The connection is also sufficiently rigid that the distance between the two electrons doesn’t change much during the lifetime of the CSS.
The researchers then loaded a solution of the test molecules into the experimental setup shown in figure
Figure 1.

Two electromagnet coils flank a sample chamber containing a solution of photoactive molecules. The surrounding optics direct a pair of laser pulses at the sample to manipulate the molecules’ spin dynamics. (Courtesy of Michael Moos.)
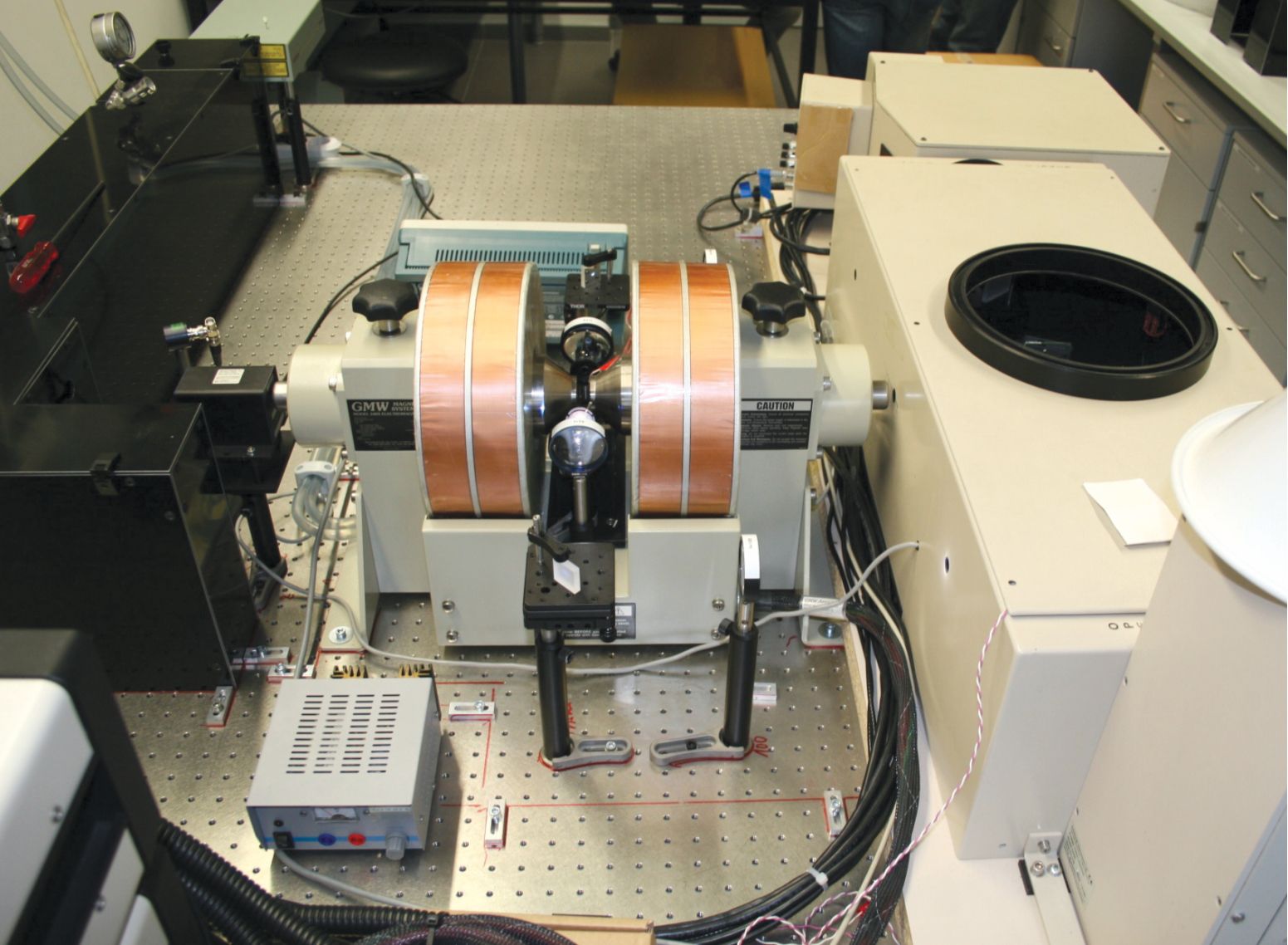
In the technique, the first pulse—the “pump”—excites an electron in the donor. From there, about 24% of the electrons fall directly back into the ground state, and the rest travel across the bridge to the acceptor and form a CSS. Those electrons also slowly relax back to the ground state over the course of hundreds of nanoseconds. But while they’re in the CSS, the electron pairs oscillate between singlet and triplet states.
A second laser pulse, the “push,” hastens the charge-separated electrons’ recombination by exciting them into still higher-energy states. Those states have extremely short lifetimes, on the order of a few hundred picoseconds. So rather than taking a leisurely trip back to the ground state, which would leave time for further spin flips, the twice-excited electrons immediately begin their return trip. And the path each electron travels down indicates which state it was in at the time of the push.
The singlets head directly back to the donor to rejoin the ground state, and along the way they produce a fluorescence signal. The triplets linger at the acceptor a bit longer in an intermediate state that is detectable by transient-absorption spectroscopy. Those electrons also eventually make it back to the donor, but only after slowly decaying through an intersystem crossing.
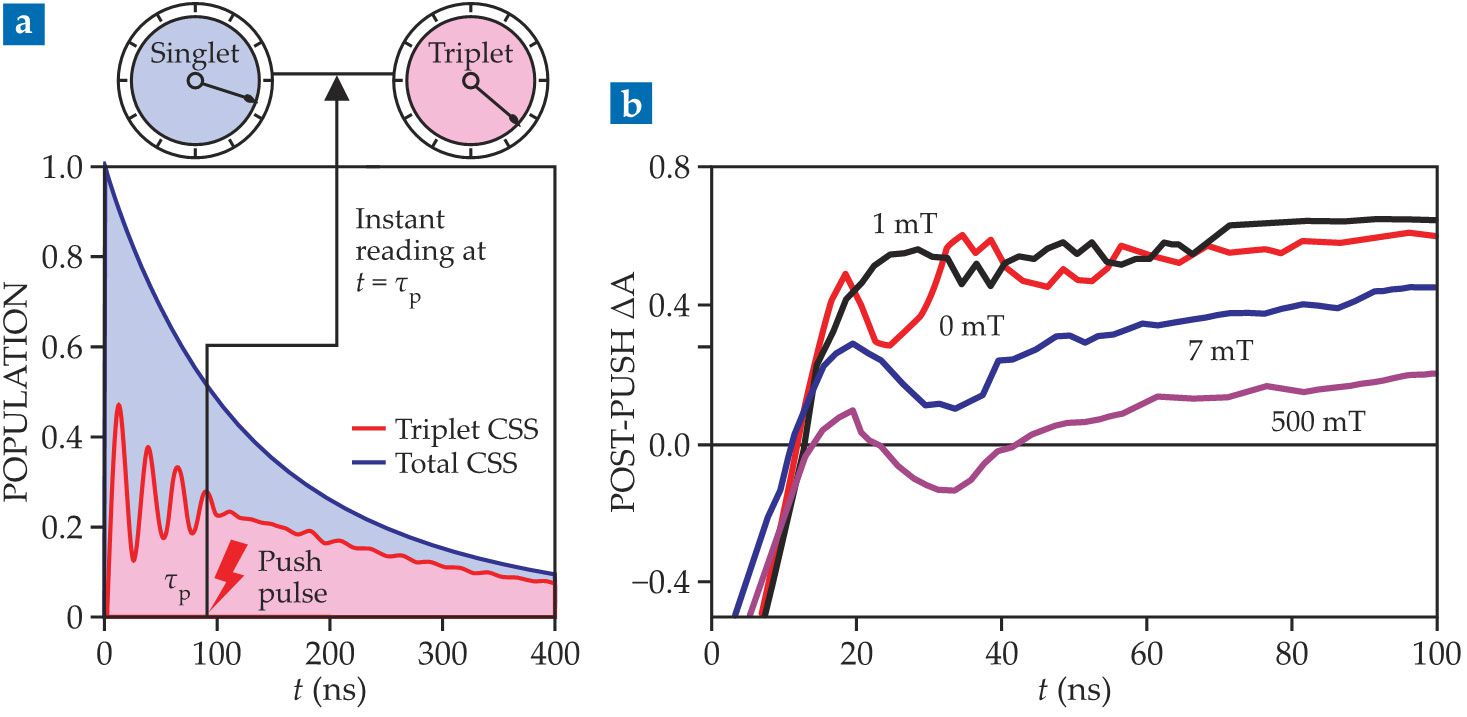
Measurements of the signals from the two spin species as they return to the ground state—fluorescence emission for the singlets and absorption for the triplets—reflect both the total population of charge-separated pairs at the time of the push pulse and the fraction of those pairs that were in each spin state. Applying the push pulse at different delay times, as illustrated in figure
Figure 2.

(a) A pump pulse at t = 0 excites electrons in specially designed test molecules such that each one hosts a charge-separated state (CSS). A push pulse applied after a time delay τp measures the fraction of the remaining states that are spin singlets and triplets. As shown schematically here, repeating the measurement with different delay times reveals oscillations that reflect the spins flipping back and forth between singlets and triplets. (b) An external magnetic field changes the frequency and amplitude of the singlet–triplet flips. Oscillations in the absorption of a triplet-decay state are plotted here at four magnetic field strengths. (Adapted from ref.
Field control
The oscillation frequencies for quantum beats in radical pairs are determined by the molecule’s internal magnetic fields. The researchers therefore designed their test molecule such that a single nucleus—a nitrogen in the triarylamine donor—dominated the effects on the molecule’s electronic structure. Otherwise, competing interactions could complicate and blur the signal, thereby making it difficult to identify and interpret.
External magnetic fields can also affect the oscillation. That gives the optical technique an advantage: Spin resonance methods can be applied at only a few discrete magnetic field values, so they can’t investigate the system’s zero-field behavior or see how its dynamics change as a function of field strength. That field restriction also limits researchers to measuring oscillations involving the triplet state with total spin ms = 0. “In general,” says Lambert, “one is interested to know about all situations of spin mixing,” including with the ms = ±1 triplet states that are accessible only at sufficiently low magnetic fields.
The transient-absorption measurements in figure
How external fields affect quantum beats is an important biological question because cryptochromes are thought to underlie migratory birds’ ability to sense Earth’s magnetic field and use it for navigation. 3 The proposed mechanism assumes that a cryptochrome’s singlet-recombination rate modulates the level of a signaling species and that the rate varies in response to the strength and direction of Earth’s magnetic field. A bird would then be able to respond to variations in the field and adjust its flight direction accordingly.
Although cryptochromes are widely suspected to underlie magnetoreception (see the article by Sönke Johnsen and Ken Lohmann, Physics Today, March 2008, page 29
To take full advantage of the new technique, researchers will have to improve its time resolution, which is currently about 8 ns—similar to the period of the test molecule’s oscillations. Biological molecules of interest are unlikely to have the test molecule’s simple spin dynamics, and their beats may happen on shorter time scales. Capturing such behavior will require a different photodetection method and shorter laser pulses. For Lambert’s lab, that would have meant costly upgrades.
Still, he intends the proof-of-principle experiments to be just the first step: “We hope that this promising novel method will be taken up by many other researchers in the field of spin chemistry and that shorter laser pulses can be applied in our or in other labs to exploit the limits of resolution.”
References
1. See, for example, A. K. Michael et al., Photochem. Photobiol. 93, 128 (2017). https://doi.org/10.1111/php.12677
2. D. Mims et al., Science 374, 1470 (2021). https://doi.org/10.1126/science.abl4254
3. See, for example, J. Xu et al., Nature 594, 535 (2021). https://doi.org/10.1038/s41586-021-03618-9




